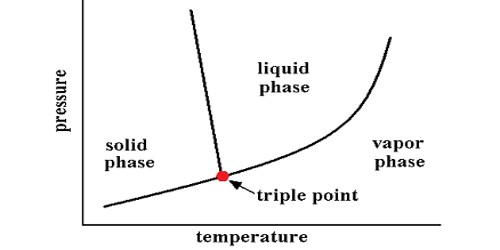
A thermodynamic study of a pure substance at the triple point is presented. In thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium. At the triple point, all three phases (solid, liquid, and gas) are in equilibrium. It is that temperature and pressure at which the sublimation curve, fusion curve, and the vaporization curve meet. The temperature and pressure at which a substance can exist in equilibrium in the liquid, solid, and gaseous states. For example, the triple point of mercury occurs at a temperature of −38.83440 °C and a pressure of 0.2 mPa.
In addition to the triple point for solid, liquid, and gas phases, a triple point may involve more than one solid phase, for substances with multiple polymorphs. The triple point of pure water is at 0.01°C (273.16K, 32.01°F) and 4.58 mm (611.2Pa) of mercury and is used to calibrate thermometers. Helium-4 is a special case that presents a triple point involving two different fluid phases (lambda point).
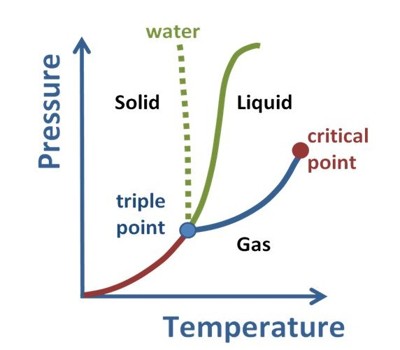
The triple point of water was used to define the kelvin, the base unit of thermodynamic temperature in the International System of Units (SI). The “triple point of water” is the point where one is interested in all “three phases” of water at a certain “pressure and temperature”. The value of the triple point of water was fixed by definition, rather than measured, but that changed with the 2019 redefinition of SI base units. The three phases include ice, water, and vapor. The triple points of several substances are used to define points in the ITS-90 international temperature scale, ranging from the triple point of hydrogen (13.8033 K) to the triple point of water (273.16 K, 0.01 °C, or 32.018 °F). It is called “triple point” because, at a particular temperature and pressure for particular substances, solid, liquid, and vapor phases coexist in equilibrium.
The term “triple point” was coined in 1873 by James Thomson, brother of Lord Kelvin. It is a specific case of equilibrium of the thermodynamic phase.