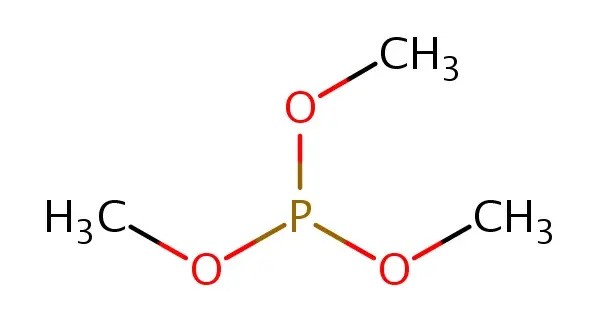
Trimethyl phosphite is an organophosphorus compound with the formula P(OCH3)3, often abbreviated P(OMe)3. It is an organophosphorus compound and is widely used in chemical synthesis and as a reagent. It is a colorless liquid with a highly pungent odor. It is the simplest phosphite ester and finds used as a ligand in organometallic chemistry and as a reagent in organic synthesis. The molecule features a pyramidal phosphorus(III) center bound to three methoxy groups.
Synthesis
Trimethyl phosphite is in principle obtainable by methanolysis of phosphorus trichloride, say in the presence of a proton accepting base. This method suffers from numerous side reactions however. The use of sodium methoxide is superior:
PCl3 + 3 NaOCH3 → P(OCH3)3 + 3 NaCl
Properties
- Chemical formula: C3H9O3P
- Molar mass: 124.08
- Appearance: colorless liquid
- Odor: distinctive, pungent
- Density: 1.052
- Melting point: −78 °C (−108 °F; 195 K)
- Boiling point: 111 °C (232 °F; 384 K)
- Solubility in water: reacts
- Vapor pressure: 24 mmHg (25°C)
Reactions
Trimethyl phosphite is susceptible to oxidation to trimethyl phosphate:
P(OCH3)3 + 0.5 O2 → OP(OCH3)3
It reacts with a catalytic amount of methyl iodide in the Arbuzov reaction to give dimethyl methylphosphonate:
P(OCH3)3 → CH3P(O)(OCH3)2
As a ligand, trimethyl phosphite has a smaller cone angle and better acceptor properties relative to trimethylphosphine. A representative derivative is the colorless tetrahedral complex Ni(P(OMe)3)4 (m.p. 108 °C).[4] The tridentate ligand called the Kläui ligand is derived from trimethyl phosphite. The formation of this ligand illustrates the susceptibility of trimethyl phosphite (and metal complexes thereof) to the Arbuzov reaction.
Occurrences and Production
Trimethyl phosphite is typically produced by the reaction of methanol with phosphorus trichloride (PCl₃):
PCl3+3CH3OH→(CH3O)3P+3HClPCl_3 + 3 CH_3OH \rightarrow (CH_3O)_3P + 3 HClPCl3+3CH3OH→(CH3O)3P+3HCl
It is not found naturally in large quantities but can be synthesized in laboratories or industrial settings for various chemical processes. It is commercially available from chemical suppliers.
Uses
- Chemical Synthesis: Trimethyl Phosphite is primarily used in the synthesis of phosphoric acid esters, phosphonates, and phosphoramidates.
- Protecting Group: It acts as a protecting group in organic synthesis, especially in the preparation of certain phosphates and phosphoramidates.
- Catalyst: It can also serve as a catalyst in some reactions involving nucleophiles or electrophiles.
- Intermediate: TMP is used as an intermediate in the production of insecticides, herbicides, and other organophosphorus compounds.
Safety Considerations
- Toxicity: Trimethyl Phosphite is highly toxic and should be handled with caution. It can cause irritation to the eyes, skin, and respiratory system. It is important to use proper personal protective equipment (PPE), such as gloves, goggles, and ventilation.
- Reactivity: TMP can be reactive, especially with water or moisture, releasing methanol and phosphoric acid.