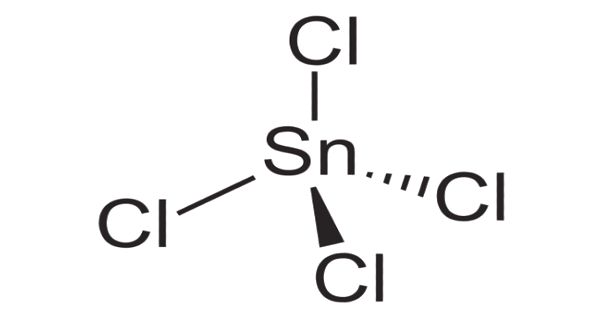
Tin (IV) Chloride is an inorganic compound. It is also known as tin tetrachloride or stannic chloride is an inorganic compound with the formula SnCl4. It is a colorless hygroscopic liquid, which fumes on contact with air. It is soluble in cold water and decomposed by hot water to form hydrochloric acid with the evolution of heat. It is used as a precursor to other tin compounds. It is corrosive to metals and tissue.
It was first discovered by Andreas Libavius (1550–1616) and was known as spiritus fumans libavii.
Tin (IV) Chloride is a colorless hygroscopic liquid, which fumes on contact with air. It is used as a precursor to other tin compounds.
Preparation
It is a strong Lewis acid widely used as a promoter or catalyst in organic synthesis. It is soluble in most organic solvents. It is prepared from the reaction of chlorine gas with tin at 115°C (239°F).
Sn + 2 Cl2 → SnCl4
Properties
- Compound Formula: Cl4Sn
- Molecular Weight: 260.52
- Appearance: Colorless Liquid
- Melting Point: −33°C
- Boiling Point: 114°C
- Density: 2.34 g/cm3
- Solubility in H2O: N/A
Structure
Anhydrous tin(IV) chloride solidifies at −33°C to give monoclinic crystals with the P21/c space group. It is isostructural with SnBr4. The molecules adopt near-perfect tetrahedral symmetry with average Sn–Cl distances of 227.9(3) pm.
Reactions
Aside from water, other Lewis bases form adducts. These include ammonia and organophosphines. With hydrochloric acid, the complex [SnCl6]2- is formed making the so-called hexachlorostannic acid.
A precursor to organotin compounds
Anhydrous tin(IV) chloride is a major precursor in organotin chemistry. Upon treatment with Grignard reagents, tin(IV) chloride gives tetraalkyltin compounds:
SnCl4 + 4 RMgCl → SnR4 + 4 MgCl2
Anhydrous tin(IV) chloride reacts with tetraorganotin compounds in redistribution reactions:
SnCl4 + SnR4 → 2 SnCl2R2
These organotin halides are more useful than the tetraorganotin derivatives.
Uses
The main application of SnCl4 is as a precursor to organotin compounds, which are used as catalysts and polymer stabilizers.
- Used as catalysts and polymer stabilizers
- Used in the preparation of SnO2 coating for toughening glass
- Used as a precursor to other tin compounds.
Information Source: