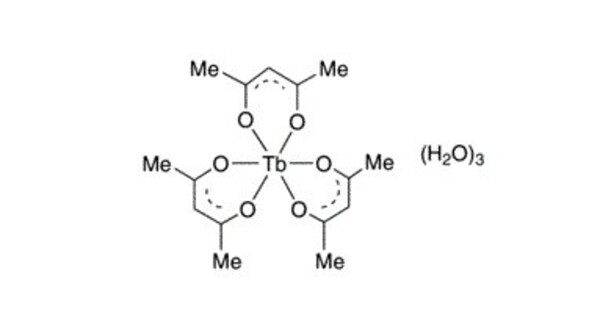
Terbium acetylacetonate is a coordination compound with the formula Tb(C5H7O2)3. It is a coordination compound of terbium, a rare earth element, with acetylacetone (also known as 2,4-pentanedione). This anhydrous acetylacetonate complex is often discussed but unlikely to exist per se. The 8-coordinated dihydrate Tb(C5H7O2)3(H2O)2 is a more plausible formula based on the behavior of other lanthanide acetylacetonates.
The dihydrate has been characterized by X-ray crystallography. Upon attempted dehydration by heating under vacuum, other hydrated lanthanide tris(acetylacetonate) complexes decompose to give oxo-clusters. The complex can be prepared from terbium salts, acetylacetone, and a base such as ammonia.
3 NH3 + 3 Hacac + Tb(NO3)3 → Tb(acac)3 + 3 NH4NO3
It reacts with 5-[(2-thiophene methylene)amino]-8-hydroxyquinoline (L) by heating in acetonitrile/dichloromethane solution to obtain light yellow [Tb(acac)4(L)6(μ3-OH)2]·CH3CN crystals. It can be used in the preparation of some optical materials.
Physical Properties
- Appearance: Usually a pale yellow or greenish solid.
- Solubility: Soluble in organic solvents such as acetone, ethanol, and chloroform, but less soluble in water.
- Melting Point: Typically in the range of 200-250 °C, but can vary depending on purity.
Chemical Properties
- Stability: Stable under normal conditions; however, it should be kept away from strong acids and bases.
- Coordination Chemistry: Acts as a bidentate ligand, forming stable complexes with terbium ions.
- Luminescence: Exhibits characteristic luminescent properties due to the f-f transitions of terbium ions, making it useful in phosphorescent materials.
Applications
- Luminescent Materials: Terbium compounds are used in phosphors for lighting and display technologies due to their luminescent properties.
- Catalysts: They can serve as catalysts in organic reactions.
- Research: Used in studies related to photonics and materials science.
Safety
As with many rare earth compounds, safety data should be reviewed before handling, as they can pose health risks if ingested or inhaled.