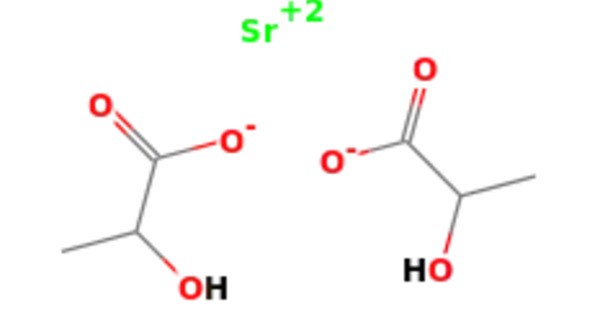
Strontium lactate is a chemical compound, a salt of strontium and lactic acid with the formula C6H10O6Sr. It is a chemical compound that consists of strontium, a metal from the alkaline earth metals group, and lactic acid. This salt is stable and non-radioactive. It is often used in various fields, including as a supplement for bone health, in the food industry, and sometimes in the production of certain types of fireworks due to its ability to produce a red color when burned.
Synthesis
Strontium lactate can be obtained by neutralizing moderately dilute lactic acid with strontium carbonate or hydroxide and evaporating the resulting solution to dryness with a moderate heat.
Occurrences
Natural Occurrence: Strontium lactate does not occur naturally as a mineral. It is typically synthesized in laboratories or industrial settings.
Synthesis: It is usually prepared by reacting strontium salts (like strontium carbonate or strontium chloride) with lactic acid in an aqueous solution.
Properties
- The compound forms white powder.
- Chemical formula: C6H10O6Sr
- Molar mass: 265.76
- Appearance: white powder
- Solubility in water: Soluble
- Chemical Stability: It is stable under normal conditions, though it may degrade or react when exposed to very strong acids or bases.
- Melting Point: It decomposes rather than melting, with the decomposition occurring at elevated temperatures.
- Reaction with Acids: Like most strontium compounds, it reacts with strong acids to release strontium salts and lactate acid.
Use
Strontium lactate is used as a dietary supplement for treating osteoporosis and supporting bones and teeth. In the food industry, it is considered a food additive (though not very commonly used) and may be included for its stabilizing properties.
In terms of health, strontium lactate is believed to play a role in promoting bone strength. It is sometimes used as a supplement in the treatment of osteoporosis or bone-related conditions, as strontium has similar properties to calcium and may help increase bone density and reduce the risk of fractures. However, it is important to consult a healthcare provider before using such supplements, as they may not be suitable for everyone.
Safety and Toxicity
While strontium is less toxic than some other alkaline earth metals like barium, it should still be handled with care. Ingesting large quantities can lead to gastrointestinal issues or bone-related problems. However, strontium lactate, in controlled doses, is generally considered safe for use in food and pharmaceutical products.