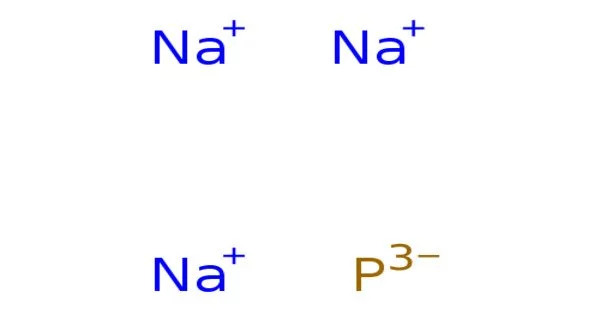The inorganic compound with the formula Na3P is sodium phosphide. It is a sodium (Na) and phosphorus (P) inorganic compound. It’s a solid black color. It is frequently referred to as the Na+ salt of the P3− anion. It belongs to the phosphide family of compounds, which are typically formed by combining a metal and phosphorus. The highly reactive phosphide anion is derived from Na3P. It should not be confused with Na3PO4, sodium phosphate.
The chemical reactivity of sodium phosphide is notable, as are its potential applications in a variety of fields. In addition to Na3P, five other binary sodium-phosphorus compositions are known: NaP, Na3P7, Na3P11, NaP7, and NaP15.
Properties
Sodium phosphide is a solid compound with a pale yellow to grayish color. It is usually found as a powder or crystalline solid.
- Chemical formula: Na3P
- Molar mass: 99.943 g/mol
- Appearance: red crystals
- Density: 1.74 g/cm3
- Melting point: 650 °C (1,202 °F; 923 K)
- Solubility in water: hydrolysis
- Solubility: insoluble in liquid CO2
Structure and Properties
The compound crystallizes in a hexagonal motif, often called the sodium arsenide structure. Like K3P, solid Na3P features pentacoordinate P centers.
Preparation
Sodium phosphide is typically prepared by reacting sodium metal (Na) with elemental phosphorus (P). The reaction is highly exothermic and should be carried out under controlled conditions.
2 Na + P4 → 4 Na3P
The first preparation of Na3P was first reported in the mid-19th century. French researcher, Alexandre Baudrimont prepared sodium phosphide by treating molten sodium with phosphorus pentachloride.
8 Na(l) + PCl5 → 5 NaCl + Na3P
Many different routes to Na3P have been described. Due to its flammability and toxicity, Na3P (and related salts) is often prepared and used in situ. White phosphorus is reduced by sodium-potassium alloy:
P4 + 12 Na → 4 Na3P
Phosphorus reacts with sodium in an autoclave at 150 °C for 5 hours to produce Na3P.
Alternatively the reaction can be conducted at normal pressures but using a temperatures gradient to generate nonvolatile NaxP phases (x < 3) that then react further with sodium. In some cases, an electron-transfer agent, such as naphthalene, is used. In such applications, the naphthalene forms the soluble sodium naphthalenide, which reduces the phosphorus.
Reactivity
Sodium phosphide is highly reactive due to the presence of the phosphide ion, which readily reacts with water to produce phosphine gas (PH3) and sodium hydroxide (NaOH).
Na3P + 3 H2O → 3 NaOH + PH3
Phosphine gas is extremely toxic and flammable, so handling sodium phosphide requires caution.
Applications
Because of its reactivity and toxicity, sodium phosphide is rarely used directly in applications. It can, however, be used to generate phosphine gas, which has applications in the semiconductor industry for doping silicon wafers and organic synthesis.
Safety Considerations
Because it emits toxic and flammable phosphine gas when it comes into contact with water, sodium phosphide should be handled with extreme caution. When working with this compound, proper protective equipment and ventilation are required.
Store sodium phosphide in a cool, dry place away from moisture and incompatible substances. To avoid accidental reactions, sealed containers and proper labeling are required.