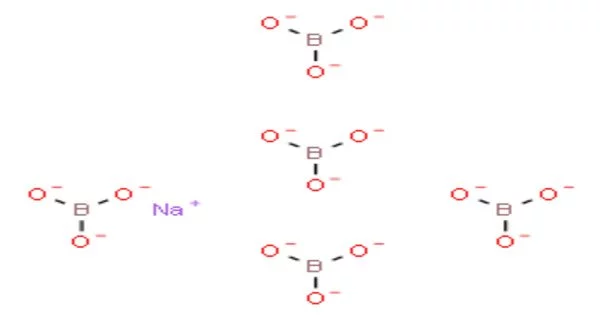
Sodium pentaborate is a chemical compound with the formula Na₅B₁₀O₁₅. It is also known as borax or sodium borate. It is a white, crystalline powder that is soluble in water. It is also known as sodium borate pentahydrate or borax pentahydrate. Sodium pentaborate is a white, crystalline solid that is soluble in water.
The compound is used in agriculture as a boron supplement in fertilizer with various trade names such as Solubor and Aquabor. It has also been tested as an additive to improve plasma electrolytic oxidation of magnesium alloys.
Properties
- Chemical formula: Na2B4O7·10H2O
- Molecular weight: 381.4 g/mol
- Melting point: 743°C (1369°F)
- Boiling point: 1575°C (2867°F)
- Solubility: Soluble in water and glycerol
- pH: Alkaline, with a pH of 9.3 in a 1% solution
- Density: 1.73 g/cm³
- Crystal system: Monoclinic
- Hardness: 2-2.5 on the Mohs scale
- Hygroscopicity: Absorbs moisture from the air
Sodium pentaborate is also a weakly basic compound that can react with acids to form salts. It has low toxicity and is generally considered safe for use in household products and as a food additive.
Application
Sodium pentaborate is commonly used in various industries such as the production of glass, ceramics, and fertilizers. It is also used as a cleaning agent, insecticide, and flame retardant. Additionally, it is used in the manufacture of soaps, detergents, and cosmetics.
Sodium pentaborate has a variety of uses, including as a component in detergents, cosmetics, and ceramics. It is also used as a buffer in chemical reactions, as a flame retardant, and as a pesticide. In addition, it is sometimes used in the production of fiberglass and in the manufacturing of some types of glass.
Toxicity
Sodium pentaborate has a low toxicity and is considered safe for most applications. However, it is important to note that sodium pentaborate can be toxic if ingested or inhaled in large quantities. It can also be irritating to the skin and eyes. Therefore, proper precautions should be taken when handling this chemical compound.