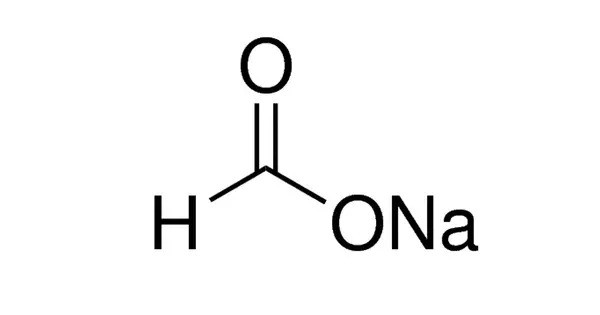
Sodium formate, HCOONa, is the sodium salt of formic acid, HCOOH. It usually appears as a white deliquescent powder. It’s commonly used in various industrial applications, such as in the production of leather, as a buffering agent in some chemical processes, and even in the production of de-icing fluids. It is also a key component in some biochemistry studies. In solid form, it appears as a white, odorless powder.
Properties
Sodium formate is soluble in water and ethanol. It has a melting point of about 253°C (487°F). It can decompose at high temperatures to release carbon monoxide (CO), and it can also react with acids to form formic acid.
- Chemical formula: HCOONa
- Molar mass: 68.007 g/mol
- Appearance: white granules deliquescent
- Density: 1.92 g/cm3 (20 °C)
- Melting point: 253 °C (487 °F; 526 K)
- Boiling point: decomposes
- Solubility in water: 43.82 g/100 mL (0 °C), 160 g/100 mL (100 °C)
- Solubility: insoluble in ether, soluble in glycerol, alcohol, formic acid
Preparation
For commercial use, sodium formate is produced by absorbing carbon monoxide under pressure in solid sodium hydroxide at 130 °C and 6-8 bar pressure:
CO + NaOH → HCO2Na
Because of the low-cost and large-scale availability of formic acid by carbonylation of methanol and hydrolysis of the resulting methyl formate, sodium formate is usually prepared by neutralizing formic acid with sodium hydroxide. Sodium formate is also unavoidably formed as a by-product in the final step of the pentaerythritol synthesis and in the crossed Cannizzaro reaction of formaldehyde with the aldol reaction product trimethylol acetaldehyde [3-hydroxy-2,2-bis(hydroxymethyl)propanal].
In the laboratory, sodium formate can be prepared by neutralizing formic acid with sodium carbonate. It can also be obtained by reacting chloroform with an alcoholic solution of sodium hydroxide.
CHCl3 + 4 NaOH → HCOONa + 3 NaCl + 2 H2O
or by reacting sodium hydroxide with chloral hydrate.
C2HCl3(OH)2 + NaOH → CHCl3 + HCOONa + H2O
The latter method is, in general, preferred to the former because the low aqueous solubility of CHCl3 makes it easier to separate out from the sodium formate solution, by fractional crystallization, than the soluble NaCl would be.
Occurrences
Sodium formate does not occur freely in nature but is commonly synthesized through the neutralization of formic acid with sodium hydroxide or sodium carbonate. It can also be produced by the reaction of sodium hydroxide with carbon monoxide under pressure (the formic acid pathway).