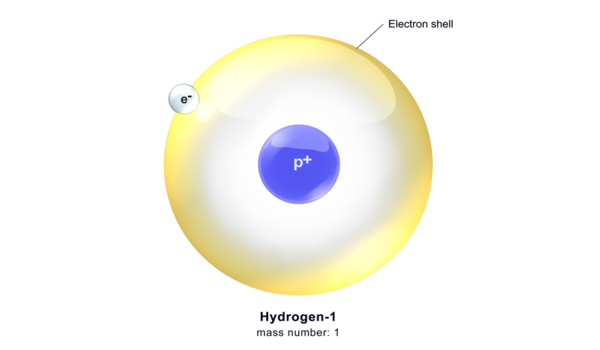
Nascent hydrogen is an antiquated idea in organic chemistry that was originally used to explain dissolving-metal reactions like the Clemmensen reduction and the Bouveault-Blanc reduction. It refers to newly formed atomic hydrogen, which is often produced by the action of an acid on specific metals. This type of hydrogen is extremely reactive due to its single, unpaired electron, making it eager to participate in chemical processes.
Nascent hydrogen is commonly employed in a variety of chemical processes, including reductions, since its high reactivity allows it to easily react with other chemicals. Since organic substances do not react with H2, a unique state of hydrogen was proposed. It is now established that dissolving-metal reactions take place at the metal surface, and the concept of nascent hydrogen has been abandoned in organic chemistry.
It is useful in organic chemistry, especially in reduction processes that transform functional groups such as ketones or aldehydes into alcohols. However, because of its high reactivity, it is typically produced in situ and employed immediately in the intended process. The creation of atomic hydrogen is frequently invoked in inorganic chemistry and corrosion studies to explain hydrogen embrittlement in metals exposed to electrolysis and anaerobic corrosion (e.g., dissolution of zinc in strong acids (HCl) and aluminium in strong bases (NaOH). Johnson proposed the mechanism of hydrogen embrittlement in 1875.
One common method to generate nascent hydrogen is through the reaction of a metal with an acid, such as the classic example of zinc reacting with hydrochloric acid:
Zn + 2HCl → ZnCl2 + H2(g)
This reaction generates hydrogen gas, and part of the hydrogen atoms created are in the nascent state. These reactive hydrogen atoms can subsequently be used for a variety of chemical reactions. However, it’s important to note that nascent hydrogen is highly reactive and frequently requires careful handling because of its ability to react aggressively with other compounds.
The inability of hydrogen atoms to react with organic reagents in organic solvents does not rule out the transient formation of hydrogen atoms capable of immediately diffusing into the crystal lattice of common metals (steel, titanium) other than those of the platinoid group (Pt, Pd, Rh, Ru, Ni), which are well known to dissociate molecular dihydrogen (H2) into atomic hydrogen.