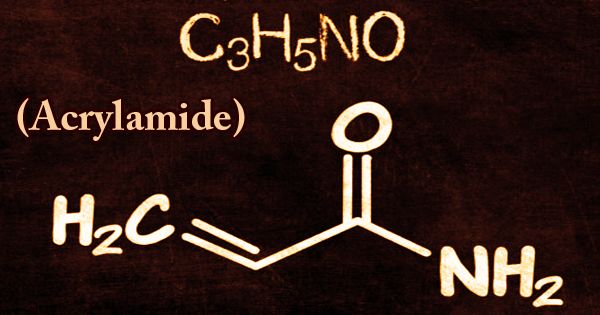
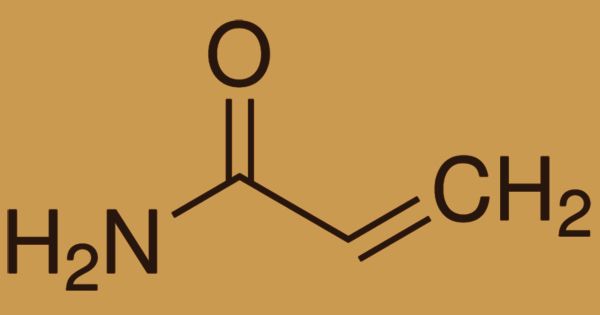
Acrylamide (or acrylic amide) is a chemical compound that occurs naturally in many foods and is commonly caused by high-heat cooking processes. It is a white crystalline chemical substance and is a raw material for polyacrylamide manufacturing, and its chemical formula is CH2=CHC(O)NH2. Strong acrylamide (AM) is typically colorless and transparent flaky crystals with a pure white crystalline solid substance that is soluble in water, methanol, ethanol, propanol, and slightly soluble in ethyl acetate, chloroform, and benzene. It can be hydrolyzed in an acidic or alkaline environment into acrylic acid. It is extremely toxic, likely to be carcinogenic, and is mostly treated as an aqueous solution, partially for that reason.

Acrylamide is a broad class of monomer parent compounds, including methacrylamide, AMPS (anionic monomer, 2-Acraylamide-2-Methyl Propane Sulfonic Acid), DMC (cationic monomer, trimethyl ammonium chloride methyl acryloyloxyethyl), and N-substituted acrylamide. The 2002 discovery that acrylamide is found in some cooked foods drew considerable attention to its potential biological effects. As of 2019, epidemiological studies show that according to IARC, NTP, and the EPA, dietary acrylamide intake is unlikely to raise people’s risk of developing cancer despite being a possible carcinogen.
By the hydrolysis of acrylonitrile, acrylamide can be prepared. Sulfuric acid as well as various metal salts catalyze the reaction. The enzyme nitrile hydratase also catalyzes it. In acrylamide processing and the synthesis of resins, adhesives, etc., occupational exposure is primarily seen. Contracts for underground building, soil development, painting, the paper industry, and the manufacturing of garments are also necessary. In some cooked foods, acrylamide occurs through a series of steps triggered by the condensation of asparagine and glucose amino acids. This condensation produces N-(D-glucose-1-yl)-L-asparagine, which generates some acrylamide upon pyrolysis, one of the Maillard reactions followed by dehydrogenation.
Acrylamide is utilized in the creation of polymers mostly in the water treatment industry, mash, and paper industry, and material treatment industry and is utilized as a lab reagent. The polymer is non-toxic, yet presented to the monomer can cause focal and fringe sensory system harm bringing about fantasies, sleepiness, and deadness in the hands and legs. It is fairly expected that acrylamide is a human carcinogen. The majority of acrylamide is used for the production of various polymers, especially polyacrylamide, which is used as a thickening agent and for the treatment of water.
Acrylamide is an unscented and dull precious stone; it is dissolvable in water, ethanol, acetone, ether, and methyl chloroform, and marginally solvent in toluene yet insoluble in benzene. Acrylamide is a water-solvent monomer with two receptive focuses. The aqueous acrylamide monomer is stabilized with dissolved cupric salts and oxygen due to its high reactivity to avoid polymerization during shipping and storage. Skin absorption toxicity; Less dense than water and water-soluble; May be harmful by ingestion. Used to produce dyes, adhesives, for sewage and waste disposal. The solid is stable at room temperature, but can violently polymerize upon melting. Toxic, irritating to the eyes, skin, etc.
Laboratory tests have shown that acrylamide is likely to be a carcinogen, the American Cancer Society notes, but that data from epidemiological studies shows that dietary acrylamide is unlikely to increase the risk of cancer in people as of 2019. People first made acrylamide using propylene chloride and ammonia at the end of the 19th century. In 1954, the American Cyanamid Corporation used acrylonitrile sulfuric acid hydrolysis for industrial manufacturing. A clearinghouse for acrylamide information has been set up by the World Health Organization (WHO) and provides a database of researchers and data providers; references to studies reported elsewhere; updates on the current status of research efforts; and updates on information relating to the health risk of acrylamide in food.
In the presence of sulfuric acid, acrylonitrile and water are hydrolyzed into acrylamide sulfate and then treated with neutralized liquid ammonia to deliver ammonium sulfate and acrylamide:
CH2 = CHCN + H2O + H2SO4 → CH2 = CHCONH2 • H2SO4 CH2 = CHCONH2 • H2SO4 + 2NH3→ CH2 = CHCONH2 + (NH4) 2SO4
Laboratory research has found that there could be the potential for certain phytochemicals to evolve into drugs that could mitigate acrylamide toxicity. The copper-based catalyst reacts with acrylonitrile with water to have a liquid phase hydration reaction at 0.4MPa pressure at 70~120 °C. CH2 = CH-CN + H2O → CH2 = CHCONH2; after the reaction catalyst, filter the catalyst; recycle the unreacted acrylonitrile; concentrate and cool the acrylamide solution to provide crystals. With a yield of up to 98%, this is an easy process.
Acrylamide is an odorless, flake-like crystal in a monomeric form that sublimates slowly at room temperature; it can be dissolved in a flammable liquid. In roasted barley tea, called mugicha in Japanese, acrylamide has been discovered. Acrylamide was found in black olives, dried plums, dried pears, chocolate, and peanuts in later tests. Based on ample evidence of carcinogenicity from research in laboratory animals, acrylamide is fairly anticipated to be a human carcinogen.
Information Sources: