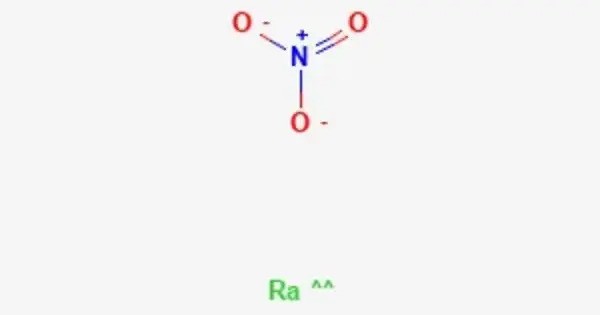
Radium nitrate is a radioactive salt with the formula Ra(NO3)2. It is a white solid, but old samples appear yellowish-grey. It is a chemical compound with the formula Ra(NO₃)₂, consisting of radium (Ra) and nitrate (NO₃) ions. It is a radioactive compound, primarily used in scientific research, particularly in radiology, as well as in studying the properties of radium.
Although radium chloride and radium bromide are less soluble than the corresponding barium salts, radium nitrate is more soluble than barium nitrate. It decomposes at 280 °C to radium oxide.
Properties
Radium nitrate is typically a white, crystalline solid at room temperature. It is soluble in water, which makes it capable of ionizing when dissolved.
- Chemical formula: Ra(NO3)2
- Molar mass: 350.01 g/mol
- Appearance: White solid
- Melting point: 280 °C (536 °F; 553 K) (decomposes)
- Solubility in water: 13.9 g/100 ml
- Solubility in nitric acid: Insoluble
Production
Radium nitrate is produced by the reaction of radium carbonate or radium sulfate with nitric acid:
RaCO3 + HNO3 → Ra(NO3)2 + CO2 + H2O
Radioactivity
It is highly radioactive due to the presence of radium, which is an alkaline earth metal. Radium isotopes decay into other elements, emitting alpha, beta, and gamma radiation. The most stable isotope of radium, Ra-226, has a half-life of 1600 years.
Radium nitrate behaves similarly to other alkaline earth metal nitrates. It is relatively stable under normal conditions but can decompose under certain circumstances, releasing nitrogen oxides (NO₂) and other products.
Occurrence
- Natural Occurrence: Radium is a rare element in nature and is typically found in trace amounts in uranium and thorium ores, particularly in pitchblende (now known as uraninite). Radium nitrate can be produced by dissolving radium compounds (such as radium carbonate or radium oxide) in nitric acid.
- Production: Historically, radium was extracted from uranium ores, especially by Marie and Pierre Curie in the early 20th century. Today, radium is primarily obtained from the decay of uranium and thorium ores.
Uses
- Scientific Research: Radium nitrate is used in the study of radioactivity and nuclear physics. It was once used in medical treatments for certain cancers before safer alternatives were found.
- Radiation Source: It has been used as a source of alpha particles in research and in applications like radiography.
- Historic Uses: In the early 20th century, radium compounds (including radium nitrate) were used in luminous paint for clocks, watches, and instrument dials, though this practice was discontinued due to the health risks associated with radium exposure.
Safety
Due to its extreme radioactivity and toxicity, the handling of radium nitrate requires strict safety measures, including the use of shielding, protective clothing, and containment procedures. Modern regulations discourage the use of radium in consumer products, and it is heavily regulated in scientific and medical contexts.