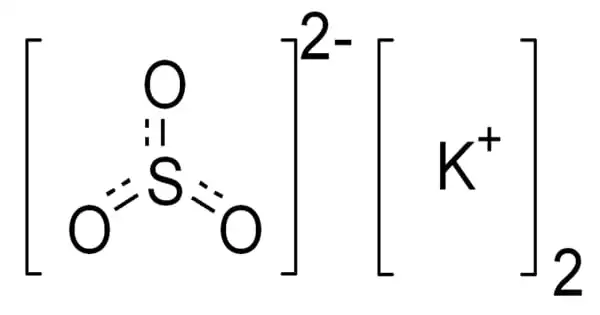
The inorganic compound potassium sulfite has the formula K2SO3. It is a potassium cation and sulfite anion salt. It is an inorganic salt that is found in a mixture with potassium hydrosulfide and is used to make pyrotechnics. It’s a white solid that’s extremely soluble in water. It is an odorless, solid white powder with a salty sulfurous flavor that is water-soluble.
It is also known as dipotassium sulfate or Sulfuric acid dipotassium salt. It can be found in volcanic lava and salt lakes. It appears as a crystalline powder or crystals that range in color from colorless to white. It has an odorless taste that is bitter, hard, and saline-like. It is water-soluble but insoluble in ethanol.
Structure
Two potassium cations K+ and one sulfide anion S2- combine to form the molecule. The two ions are joined together by an ionic bond. The molecule has an antifluorite structure, with a cation K+ surrounded by eight sulfide anions.
Properties
- Molar Mass: 174.259 g/mol
- Density: 2.66 g/cm³
- Boiling Point: 1,689 °C
- Melting Point: 1,069 °C
- Molecular Weight: 158.26
- Appearance: White powder

Production and reactions
Potassium sulfite is produced by the thermal decomposition of potassium metabisulfite at 190°C:
K2S2O5 → K2SO3 + SO2
The oxidation of aqueous sodium sulphite is an auto-oxidation that demonstrates the aforementioned initiation mechanisms. It’s a classic chain reaction. It is extremely light-sensitive, with a quantum yield of about 105. It is also sensitive to the presence of some organic compounds, such as aniline, carbohydrates, or glycerol; any of these, in small amounts, renders the sulphite solution oxygen stable.
The steps followed to obtain this compound are as follows:
- Crushing the mineral langbeinite
- Washing it
- Extracting the mineral
- Separating
The product is then treated with an aqueous potassium chloride solution to separate the two parts of the double salt. Synthetic potassium sulfate compounds are also available. This is accomplished by reacting potassium chloride with undiluted sulfuric acid.
Potassium sulfate is a potassium source for plants. It is a type of potash fertilizer that is widely used. Potassium (K) is an essential nutrient for plant growth and is particularly important for food crops. Potassium, also known as potash, helps plants use water, resist drought, and improve the flavor of fruits and vegetables.
Use
Potassium sulfite is widely used in food and beverage preservation. It is used as a pyrotechnic component. It is primarily used in the production of the fireworks senki hanabi in Asia. Potassium sulfide is also used in the manufacturing of glitter.
Its primary applications are as a food preservative and as an antioxidant. Sodium sulfite was once used to make pulp in the paper industry, but it now accounts for less than 10% of total pulp production, and the number of sulfite mills is declining. In addition, sodium sulfite is used in water treatment and photography.
- It is used in soda blasting.
- It is used as a supplement for animal feeds.
- It is used in the production of lubricants and dyes.
- It is used in the manufacturing of ceramics and glass.
- It is used in the production of gypsum board.
- It is used to produce gypsum cement.
- It is used in explosives as a flash suppressant
Health effects/safety hazards
It is hazardous to one’s health. Toxic fumes produced by heating are hazardous to inhalation. It is extremely flammable, and its particles can combine to form explosive mixtures in the air, igniting spontaneously.