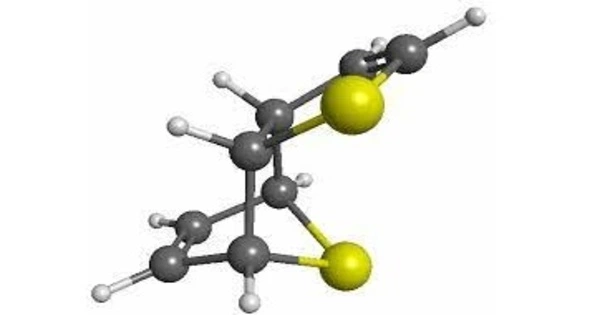
A carbon nanothread (also known as a diamond nanothread) is a one-dimensional carbon crystalline nanomaterial that is sp3-bonded. Its carbon has a tetrahedral sp3-bonding structure similar to diamond. Nanothreads are a few atoms wide and 20,000 times thinner than human hair. They are made up of a strong, stiff carbon core surrounded by hydrogen atoms.
A team of scientists from the Center for High-Pressure Science and Technology Advanced Research (HPSTAR) led by Drs. Kuo Li and Haiyan Zheng reported the first synthesis of a three-dimensional crystalline carbon nanothread (CNThs) from a biomass precursor, 2,5-furandicarboxylic acid (FDCA), via [4+2] Diels-Alder reactions.
Crystalline CNTh shows excellent electrochemical performance as an anode material for lithium batteries. The work is published in the Journal of the American Chemical Society.
The reaction pressure of 12GPa is relatively low. This is also the first time that biomass compounds have been used to synthesize diamond carbon nanothread materials. We believe that our research will open up a new avenue for the creation of advanced functional carbon materials.
Dr. Haiyan Zheng
Carbon nanothread is a one-dimensional diamond material with high tensile strength and modulus of bending. Scientists synthesized CNTh for the first time in 2015 using pressure-induced polymerization of benzene. Later, a series of CNThs were synthesized from various aromatic molecules. However, how to improve the atomical intra- and interthread ordering has long been a source of contention for CNTh.
FDCA is one of the top twelve value-added chemicals derived from sugar and is widely used in the chemical industry. “It has fewer possible bonding routes than benzene, which will help to improve CNThs homogeneity. Furthermore, its aligned – stacking and intermolecular H-bonding in its structure are important for obtaining the crystalline diamond nanothread. As a result, 2,5-furandicarboxylic acid is a better CNTh precursor material” Dr. Kuo Li stated.
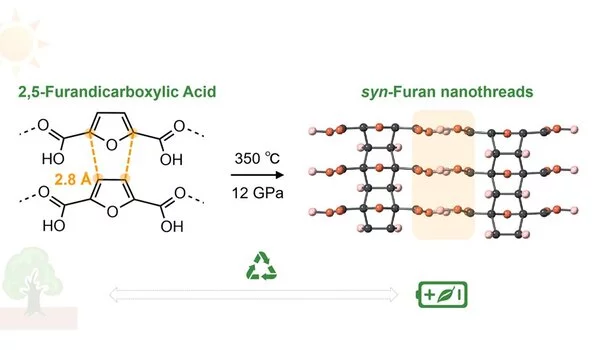
In this work, the scientists synthesized the atomically ordered crystalline CNTh with uniform syn-configuration (all the oxygens on one side) by compressing FDCA at ~12 GPa. With the exceptional long-range ordering in the product, they determined the precise crystal structure directly from the X-ray diffraction, showing an obvious contrast to the previous reports of CNThs.
The FDCA underwent continuous [4+2] Diels-Alder reactions along the stacking direction, according to spectroscopy and theoretical simulation. Because of the abundance of carbonyl groups, poly-FDCA CNTh has a high specific capacity, excellent coulombic efficiency, and rate performance as a Li-battery anode material.
“The reaction pressure of 12GPa is relatively low,” said Dr. Xuan Wang, the study’s lead author. “This is also the first time that biomass compounds have been used to synthesize diamond carbon nanothread materials. We believe that our research will open up a new avenue for the creation of advanced functional carbon materials” Dr. Haiyan Zheng also contributed.