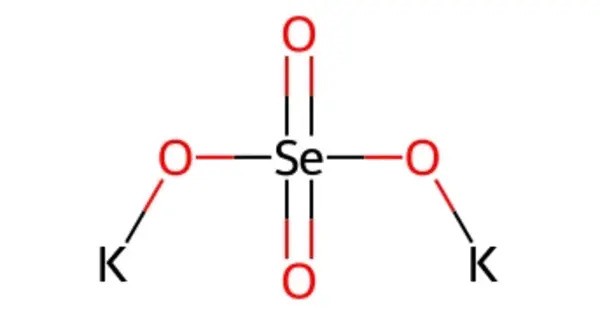
Potassium selenate, K2SeO4, is an odorless, white solid that forms as the potassium salt of selenic acid. It is an inorganic salt that is often used in various industrial applications, including as a precursor to selenium compounds in the production of semiconductors and other electronic devices. Potassium selenate is also sometimes used in agriculture as a source of selenium for plants, though it must be used carefully, as selenium can be toxic in high concentrations.
Preparation
Potassium selenate is produced by the reaction of selenium trioxide and potassium hydroxide.
SeO3 + 2 KOH → K2SeO4 + H2O
Alternatively, it can be made by treating selenous acid with potassium hydroxide, followed by oxidation of the resulting potassium selenite with bromine water.
H2SeO3 + 2 KOH → K2SeO3 + 2 H2O
K2SeO3 + 2 KOH + Br2 → K2SeO4 + 2 KBr + H2O
Properties
Potassium selenate appears as a white, crystalline solid. It is soluble in water, which makes it reactive in aqueous solutions. It is considered an oxidizing agent. This means it can donate oxygen atoms to other substances, which is an important feature in chemical reactions.
- Chemical formula: K2SeO4
- Molar mass: 221.2 g/mol
- Appearance: colorless crystals hygroscopic
- Odor: odorless
- Density: 3.07 g/cm3
- Solubility in water: 1.07 g/ml (0 °C), 1.11 g/ml (20 °C), 1.22 g/ml (100 °C)
Occurrences
Potassium selenate can be found in trace amounts in nature, primarily in soils, where selenium can be present in different forms, including as selenates and selenites. Some plants and animals can absorb selenium from the soil, though in high concentrations, selenium can be toxic.
While it’s not typically found in large natural deposits, it can be synthesized industrially. Potassium selenate is often produced by oxidizing selenium compounds such as sodium selenite (Na₂SeO₃) with potassium hydroxide (KOH) in the presence of oxygen.
Uses
Potassium selenate can be used to produce selenium trioxide. It can also use to treat selenium deficiency in livestock.
- Agriculture: It can be used as a micronutrient in fertilizers to provide selenium to plants, which is essential for some forms of life but needed only in small amounts.
- Analytical Chemistry: It’s used in chemical analysis and synthesis.
- As a source of selenium: Because selenium is an essential element in trace amounts for both plants and animals, potassium selenate can be used to enrich food supplies and animal feeds.
Toxicity
Like many selenium compounds, potassium selenate can be toxic if ingested in large quantities, though selenium is also an essential trace element in the diet. However, high concentrations can lead to selenium poisoning, affecting various systems in the body.