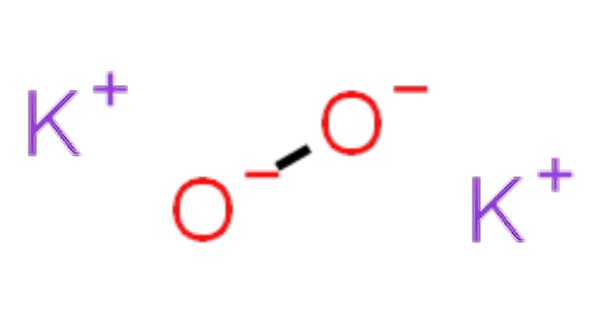
Potassium peroxide is an inorganic compound with the molecular formula K2O2. It typically appears as a yellowish or white solid and is known for its strong oxidizing properties. It is formed as potassium reacts with oxygen in the air, along with potassium oxide (K2O) and potassium superoxide (KO2).
Potassium peroxide reacts with water to form potassium hydroxide and oxygen:
2 K2O2 + 2 H2O → 4 KOH + O2 ↑
In contact with moisture or water, potassium peroxide can decompose, releasing oxygen gas and forming potassium hydroxide (KOH). This makes it potentially hazardous, as it can cause fires or explosions in the presence of flammable materials or when exposed to water.
Properties
Potassium peroxide is a highly reactive, oxidizing white to yellowish solid which, while not flammable itself, reacts violently with flammable materials. It decomposes violently on contact with water. The standard enthalpy of formation of potassium peroxide is ΔH f 0 = −496 kJ/mol.
- Chemical formula: K2O2
- Molar mass: 110.196 g/mol
- Appearance: yellow amorphous solid
- Melting point: 490 °C (914 °F; 763 K)
- Solubility in water: reacts with water
- Crystal structure: Orthorhombic
Occurrences
- Synthesis: Potassium peroxide is generally synthesized in the laboratory or industrial settings by the reaction of potassium hydroxide (KOH) with oxygen, or by heating potassium nitrate (KNO₃) with a reducing agent.
- Natural Occurrence: It’s not commonly found in nature in large quantities but might appear in small amounts in certain mineral deposits, especially those involving potassium and oxygen. It can form in volcanic areas due to the high amounts of oxygen available.
Usage
Potassium peroxide is used as an oxidizing agent and bleach (due to the peroxide), and to purify air.