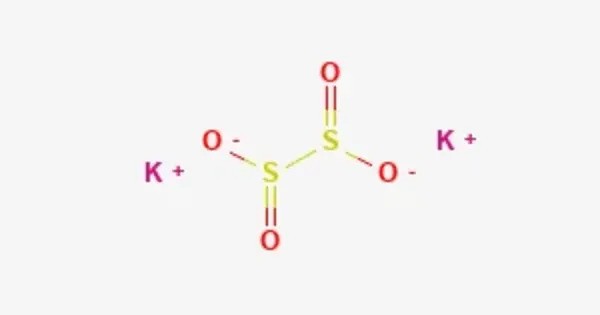
Potassium dithionite or potassium hydrosulfite is a potassium salt of dithionous acid. It is an inorganic compound, also known as potassium hydrosulfite. It is a white crystalline solid with strong reducing properties, making it valuable in industrial and laboratory applications. The compound is moderately soluble in water and decomposes rapidly in aqueous solutions, especially under acidic conditions, releasing sulfur dioxide and other sulfur-containing species.
The compound has UN number UN 1929. As a dithionite, it is closely related to sodium dithionite, which is a commonly used reducing agent and bleaching agent.
Properties
- Chemical formula: K2O4S2
- Molar mass: 206.32 g/mol
- Appearance: light yellow powder
- Solubility: Soluble in water, decomposes rapidly in acidic solutions
- Stability: Unstable in air, especially in moist conditions; decomposes releasing sulfur dioxide (SO₂)
- Reducing agent: Strong, used for reducing various compounds, similar to sodium dithionite but less common
Occurrences
- Natural occurrence: Does not occur naturally due to instability; always prepared synthetically.
- Production: Obtained by reacting sulfur dioxide with potassium hydroxide, or via reduction processes involving potassium bisulfite.
Applications
As a powerful reducing agent, potassium dithionite is commonly used in textile industries for bleaching and dyeing processes, particularly for vat dyes and indigo, where it reduces insoluble dye molecules into soluble forms. It is also employed in the paper and pulp industry as a bleaching agent, offering an alternative to chlorine-based processes. In organic synthesis, it serves to reduce nitro compounds, quinones, and certain metal ions.
Safety
Potassium dithionite is less commonly used than its sodium counterpart (sodium dithionite), due to stability and handling differences, but it offers similar chemical reactivity. The compound must be stored carefully, as it is sensitive to heat, moisture, and air, which can trigger decomposition and loss of effectiveness.