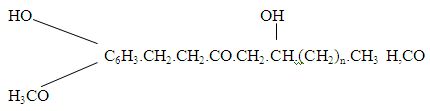Zingiber Officinale Roscoe belongs to the family of Zingiberaceae in the order Zingiberales.The family Zingiberaceae of the sub-order Scitaminae is comprised of some 40 genera and hundred of species.The genus Z.Boehmer is the most important of which is represented by Z. Officinale Roscoe,yields ginger and is grown extensively in many tropical countries.It is a herbaceous, rhizomatous, perennial plant, producing leafy shoots, which attains a height of about 1 to 3 ft. Rhizomes are robust, aromatic, thick lobed, pale yellowish, different shape and size in the different cultivated types.The rhizomes borne horizontally just below the soil surface. The fleshy sympodial rhizome is hard and thick, some what laterally compressed, often palmately branched and with fine fibrous roots on the top layers of the soil. It is pale brown in colour externally and greenish yellow within.
The herb develops several lateral shoots in clumps which begin to dry when the plant matures. Leaves are narrow, distichous, sub – sessile, linear – lanceolate, 17cm X 1.8 cm, dark green, evenly narrowed to form a slender tip, flowers in spikes, greenish yellow with a small dark purple or purple or purplish black tip.
Zingiber Officinale Roscoe is the most important of all the spices obtained from the underground plant part. It was also one of the first oriental spices to be grown to the Europeans. But now it is found to grow extensively in the tropical and sub – tropical regions of the world particularly in Bangladesh, India, Taiwan, Jamaica, Africa,Mexico, Nigeria, Sierra Leone, China and Japan.
It is well known that ginger rhizomes contain both aromatic and pungent components.The essential oil and oleoresins extracted from ginger rhizomes are very valuable product responsible for the characteristic of ginger flavor and pungency.Both oil and oleoresins are used in many food items, soft drinks, beverages and many types of medicinale substances.
Scientific Classification of Zingiber officinale Roscoe
Kingdom : Plantae
Sub-Kingdom : Tracheobionta
Super division : Spermatophyta
Division : Magnoliophyta
Class : Liliopsida
Sub-class : Zingiberidae
Order : Zingiberales
Family : Zingiberaceae
Genus : Zingiber
Species : Z. officinale
Binomial name : Zingiber officinale
Synonyms : Amomum Zingiber L.
Description of the herb Zingiber officinale Roscoe:
Ginger is a perennial herb.Ginger requires a warm and humid climate.This crop can thrive well in sandy or clayey loam or lateritic loam soils. It is often cultivated as warm weather crop.It grows best in well watered soil in partial shade.
Stem:
Ginger plant is a perennial herb.Leafy stem 0.5-1.0 m high. Leafy stem covered with leaf sheath of the lanceolate-oblong to linear leaves.
Leaves:
Ginger leaves are sessile, linear-lanceolate, narrow, lance-shaped, long-acuminate, glabrous or puberulous along midrib beneath, ligule 2-4 mm long, membranous, shallowly bilobed. Spike radical 4.5-7.0 ´ 2.0-2.5 cm ovoid.
Flowers:
The sterile flowers are white with purple streaks and grow in spikes. Peduncle 10-20 cm long.Corolla tube 2.2-2.5 cm long. Petals Creamy yellow, dorsal c. 20´8 mm, narrowed at apex, laterals c. 17´5 mm narrowed at apex.
Fruits:
Fruits are red in colour. Each has three chambers containing several small black seeds. Ginger plants that are cultivated in commercial plantations don’t usually bear fruit.
Root or Rhizome:
Rhizomes knobbly and fleshy, covered in ring-likes scars. This is the important part for food and medicine. Although the rhizomes grow underground, they are swollen stems, not roots. This is why fresh ginger is often referred to as stem ginger.
Used Part:
Rhizomes are mainly used as spice but it has a great use in medicine and its herb can also use as the same.
Habitat and range:
Zingiber officinale Roscoe (Ginger) is one of the 5 most important major species of the genus Zingiber belonging to the family Zingiberaceae. It is a perennial herb. According to available historical records, ginger was certainly known to and highly esteemed by the ancient Greeks and Romans who obtained this spice from Arabian traders via the Red Sea. It was introduced to Germany and France in the 9 th century and to England in the 10 th century.This specie is planted almost all over the country under cultivated condition.But ginger is cultivated commercelly in Chittagong, Mountanious Chittagong and Rangpur.
It is also found to grow in the tropical and sub-tropical Asia. Ginger cultivation began in south-Asia and has since spread to East-Africa and the Caribbean and Jamaica, Japan, China, Taiwan and Australia.In Bangladesh, ada is grown in Rangpur, Chittagong, Mountanious Chittagong, Soadpur, Gajipur(BARI) and it is also grown in experimental field of Spice Research Center, Bogra.
Plant History:
Botanical names are often derived from their ancient names. The word Zingiber is such an example. It is thought to come from the Sanskrit word Singabera which was from Arabic and Greek word meaning ‘shaped like a horn’. It probably got its name because the rhizomes look like deer’s antlers. Ginger is a perennial plant, the aromatic, knotty rootstock, thick, fibrous, and whitish or buff-colored. It produces a simple, leafy stem covered with the leaf sheaths of the lanceolate-oblong to linear leaves. The plant reaches a height of 3-4 feet, the leaves growing 6-12 inches long. The upright shoots sprout from the rhizome at the base of the plant.
Plantation of Zingiber officinale Roscoe Plant:
a) Cultivated Types:
Several commercial types are recognized in cultivation. They are generally named after the localities where they are grown. The types with less fibre, which varies from 1.7 to 9.0 percent, have higher demand for cultivation.
b) Climate and Soil:
The plant requires a tropical or subtropical climate where the temperature is high for at most part of the year. Bright sunshine, as well as heavy rains are necessary.
The most suitable soil consists of a high, free, sandy loam. Stiff clays on coarse sand are not conducive to the cultivation of the plant. Sandy soils are apt to peek after a heavy rain and to become too dense for the rhizomes to develop. Wet swampy ground does not suit for the plant at all, and areas liable to hoods should be avoided. If the ground becomes too dry during the dry season, a system of irrigation will be needed.
c) Seasons:
According to Ridley, planting of ginger in Bangladesh and India, generally takes place in March and April – though occasionally until June depending upon the time of onset of the wet season.
d) Preparation of Land and Cultivation:
Prior to planting, the soil must be broken up finely with a hoe or plough, and if possible harrowed afterward. The ginger is usually grown from cuttings of the rhizome. The optimum spacing for planting of ginger is 25-45 cm. between the rows and 15-20 cm. between the plants in row. The seed rhizome is harvested between 5 and 7 months after sowing. The seed rhizome with at least two sprouted eychuds are placed 3.5-5.0 cm deep in the pits and the soil is pressed over it; this is followed by light irrigation.
Under favourable conditions the ginger plant appears above the ground about ten to fifteen days after planting. But as much as two months may be required before it begins to show. The rhizomes can be lifted from the ground (harvested) when the green leafy stems turn yellow and within. This usually happens after the flowering period. In general, the rhizomes mature within nine to ten months after planting. The matured rhizomes are lifted from the soil by a single thrust of the fork. Then the rhizomes are piled into heaps, the roots broken off and soil and other adhering matters are removed.
e) Storage of seed rhizome:
The seed – rhizomes is to be stored from the time of harvesting in December to the time of sowing during April – May, a period of 120-150 days, but is a highly perishable item and is susceptible to attack by soil borne fungi, insects, and white ants. The seed rhizome which are well – developed and disease – free are treated with 0.25 percent solution of Wet table Cereasan for 30 minutes as a prophylactic measure against soft rot. The treated rhizomes are dried under shade to remove excess moisture. The dried rhizomes are loosely stored in pits, which is about a meter in depth in a cool place. A layer of sand or saw – dust is spread at the bottom of the pit and seed – rhizomes are filled in it.
Chemical composition of Ginger:
The composition of ginger varies according to the type and agro – climatic conditions & under which it is grown. Analysis of a bazar sample of green ginger gave the following values: Moisture, 80.9; Protein, 2.3; fat, 0.9; fiber 2.4; carbohydrates, 12.3; and minerals, 1.2%; Ca 20; P, 60 and Fe 2.6 mg. / 100 g., Ginger contains traces of idodine and fluorine (J, 0.82 ppm. and Fe 2.0 ppm in one sample). The vitamins present in ginger are: thiamine, 0.06; riboflavin, 0.03; niacin, 0.60; and vitamin C, 6.0 nig / 100g. The value reported for carotene in the fresh rhizome is 40hg/100g. Ginger contains small guantilics of glucose, fructose and sucrose; raffinosc is probably preset in traces. The principal carbohydrate of rhizome is starch. Ginger contains 1.60 – 2.44 per cent nitrogen on dry basis, of which non – protein nitrogen accounts for roughly one – third. The free amino acids present in ginger include serine, glycine, threonine, alanine, glutamine, arginine, 5 – aminobutyric acid, valine, henylalanine, asparagine, lysine, cystine, histidine, leucines, proline and pipecolic acid.
Chemical composition of ginger essential oil:
The characteristic of pleasent and aromatic odour of ginger is due to an essential oil. Ginger contains form 0.25 to 3 percent of volatile oil of light yellow colour. The oil is sparingly soluble in 95 percent alcohol, but is generally soluble in 90 percent alcohol.
The oil contains sesquiterpene hydrocarbons (50% on more), aesquilerpene alcohols, monoterpenoids and associated compounds, the occurrence of eslcrs of acetic and caprylic acid and a trace of phenol.
The oil of ginger mainly contain terpenes (d-camphene, and β – phellandrene), a sesquiterpene (zingiberene), cineole, withal and borneol. The predominat esquiterpene hydrocarbon is Zingiberene (α and β- Zingiberene) (C15H24), the principal constituent of ginger oil. Other sesquiterpenes present in the oil are ar – curcumene (17.7%) farnesenc (9.8%) and relatively small amounts of β-bisabolene,citronellal, α-terpineol,neral, δ selinene, β-elemene and β-sesquiphellandrene. The sesquiterpene alcohol, zingiberol (C15H26O), which is a mixture of β- cudcsmol stereoisomers, occure in the oil.
The monoterpene hydrocarbons present in the oil include camphcnc, α and β pincnc, myrcene, limonene, p- cymene and β- phellandrene. The oxygenated monoterpenes and associated compounds present are 2 – heptanol, 2 – nonanol, n – nonanal, methyl hcptcnone; 1,8- eineole, borneol, borxyl acetate, lixalool, gcraxial and ncral. The pungency of ginger is due to the “Oleo – resin”, gingcrol, Zingeronc on oily hiquid consisting of homologous phenols, of this type:
a) In Medicine:
Ginger is mostly used for the purpose of manufacturing various types of medicine. Ginger is well known as a remedy for travel sickness, nausea and indigestion and is used for wind, colic, irritable bowel, loss of appetite, chills, cold, flu, poor circulation, menstrual cramps, dyspepsia (bloating, heartburn, flatulence), indigestion and gastrointestianl problems such as gas and stomach cramps. Ginger is powerful anti-inflammatory herb and there has been much recent interest in its use for joint problems. It has also been indicated for arthritis, fevers, headaches, toothaches, coughs, bronchitis, osteoarthritis, rheumatoid arthritis, to ease tendonitis, lower cholesterol and blood-pressure and aid in preventing internal blood
clots. Ginger has been well researched and many of its traditional uses confirmed.Ginger root is a mal herb used primarily for the treatment of Dyspepsia (discomfort after eating), this includes the symptoms of bloating, heartburn, flatulence, and nausea. It is also consihelpful as a preventative for motion sickness and as a digestive.Due to it’s antispasmodic characteristic some people have used it to help ease menstrual cramps.In some traditional systems it is credited with the ability to treat arthritis, fevers, headaches, and toothaches.Ginger may also be taken orally as a herbal remedy to prevent or relieve nausea resulting from chemotherapy, motoin sickness, pregnancy, and surgery. There are some evidence to suggest that it helps to combat skin, ovarian, and breast cancer.
In general no adverse effects were noted from using ginger for eaither the mother or developing baby.
b) Other uses of ginger:
In Food Preparations:
The aroma of ginger is pleasant and spicy and its flavour penetrating, slightly biting due to antiseptic or pungent compounds present in it, which make it indispensable in the manufacture of a number of food products like ginger bread, stationery, ginger ale, curry powders, certain curried meats, calorific sauces, in pickling and in the manufacture of certain soft drinks. It is like cordials, ginger cocktail, carbonated drinks, bitters; type ginger is also used for the manufacture of ginger oil, oleoresin, essences, tinctures, etc.
Ginger Candy/Preserve:
Ginger Preserve and Ginger Candy prepared from green or fresh ginger are quite a favourite of many find great demand.
Alcoholic Beverages:
A number of alcoholic beverages are prepared from ginger in foreign countries, such as ginger brandy, ginger wine, ginger beer and ginger ales, etc.
As Flavourant:
Ginger oil is primarily used as food flavourant in soft drinks like ginger ale, bitters, cordials and liquors, as a spice in bakery products, confectionery, pickles, sauces and preserves, etc.
Pharmaceutical use:
The Pharmaceutical uses are carminative, rubefacient, stimulant, in alcoholic gastritis, dyspepsia, flatulent colic, etc. Veterinary uses of ginger are as stimulant and carminative, in indigestion of horses, and cattle, in spasmodic colic of horses, and to prevent the griping by purgatives.
Use in perfumery:
The oil of ginger finds limited use in perfumery, where it imparts a unique individual note to compositions of the oriental type.
Objective of the Research Program:
The land of Bangladesh is very fertile.There are many kinds of plants, herbs and trees. Various herbaceous medicinal and aromatic plants have been used for medicinal, spices and flavoring purpose in the world wide. Zingiber officinale Roscoe (Ginger) is a popular perennial herb.Which is cultivated more or less almost all over the country. The composition of plant based product mostly depends on the agro climatic condition (climate and soil) of the country. The chemical composition of the Ada herbs varies with the agro climatic conditions of the part of the country where it is grown.
Moreover many research have been carried out on Zingiber officinale Roscoe but no systematic research has been carried out on it in Bangladesh. Some disagreement about the presence of its constituents was observed. Therefore the present work was undertaken to carry out a complete investigation on the Zingiber officinale Roscoe (Ginger).
The followings are the aims and objectives of the study:
1) To carry out the complete study and to get information for consumption and utilization as well as industrial application of this plant and its product.
2) To observe its popularity consumption and availability as a perennial herb and condiment (its rhizome) as well as to explore export market to the foreign countries.
3) Characterization quantification, and identification of the constituent compounds of the essential oil isolated by steam distillation from its seed by GC-MS (gas chromatography and mass spectroscopy).
4) To investigate the mineral contents including toxic heavy metals and nutrients which remained after extraction of essential oil as well as fatty oil of rhizome by Atomic absorption spectrometer (AAS).
5) A comparative study on the composition of Zingiber officinale Roscoe (Ginger) rhizome collected from different regions of Bangladesh.
6) To carry out the complete study on the nutrients of Zingiber officinale Roscoe (Ginger) rhizome.
Scheme of the work:
Zingiber officinale Roscoe (Ginger) rhizomes were collected from different parts of Bangladesh. Following experiment were undertaken for complete investigation on Ada rhizome.
1. Essential oil part:
- Extraction of Essential oil by means of steam distillation.
- Determination of Physical Properties & Chemical Properties of Essential Oil.
- Antioxidant activity of Zingiber officinale Roscoe (Ginger) .
- Antimicrobial Screening of Zingiber officinale Roscoe (Ginger).
- Cytotoxicity Assay of Zingiber officinale Roscoe (Ginger).
- Analysis of the oils by GC-MS for identification and quantification of its constituent compounds as well as their structures.
2. Fatty oil part:
- Soxhlet Extraction of fatty oil.
- Determination of Physical and Chemical properties of the fatty oils.
- Determination of Physical Properties & Chemical Properties of Essential Oil
3. Residual part:
- Determination of moisture, ash, crude fiber, nitrogen, protein, carbohydrates content and food energy of the rhizome.
- Determination of mineral content of rhizome the herb by AAS (Atomic absorption spectrometry).
- Identification of toxic and useful trace elements contained in the rhizome.
- IR analisis of Zingeber Officinale Roscoe (Ginger)
Essential Oil
Volatile oils or Essential oil:
The term “Essential oil” defined as the volatile oil obtained by the steam distillation of ginger rhizome. With such a definition it is clearly intended to make a distinction between the fatty oils and the oils which are easily volatile. Their volatility and rhizome origin are the characteristic properties of these oils. Essential oil is any of a class of volatile oils composed of a mixture of complex hydrocarbon (usually terpenes) and other chemicals extracted from ginger rhizome. Essential oils are characterized by their capacity to generate a flavor or aroma and taste.
All distinctly aromatic plants contain essential oils and these plants are found in some sixty families, which are distributed throughout the PlantKingdom. Certain families, however, are noted for their aromatic members and, in fact, the possession of aromatic oils is a characteristic of some families. Such additional families as the laurels, wax myrtle’s and the daisy family also contain numbers of aromatic members.
Essential or volatile oils are byproducts of metabolism, which have become useful to the plant only secondarily. The oils are never in Free State in living cells but are deposited in various types of cavities throughout the plants body. These Storage spaces may consist of dead rhizome, cavities formed by the disintegration or shrinkage of calls or especially formed capsules, and they are found in any or all parts of the plant, such as root or rhizome(pale yellow),stem(sprour),leaves(sessile),petals(creamy yellow) flower(yellow). They are generally liquids but may be semi solids or solids.
Gradually with the advance of science came improvement in the methods of preparing the oils, and parallel with this development a better knowledge of the constituents of the oils was gained. If was found that the oils contain chiefly liquid and more or less volatile compounds of many classes of organic substances. This we find acyclic and cyclic hydrocarbons and their oxygenated derivatives. Some of the compounds contain nitrogen and sulfur. Although a list of all the known oil components would include a variety of chemically unrelated compounds, it is possible to classify a large number of these into four
main groups, which are characteristic of the majority of the essential oils. Representatives of this last group are incidental and often. Rather specific for a few species (or genera). Most volatile oils are lighter than water and dissolve in it to slight extent. Some are heavier than water, e.g. oils of wintergreen, sassafras, cinnamon, mustard, clove, etc.
Collection of two Varieties Ginger:
The Bangladeshi Ginger and China Ginger are available in the local markets of DhakaCity. Here China Ginger is available because a large quantity of Ginger are imported from China.
Cleaning/Drying and Chipping:
The collected samples were washed clearly by water to remove dust materials. Then they were dried. Finely the dried ginger was reducing to 2 – 5 cm flat chips with heavy knives.
Extraction of essential oils:
There are a number of methods employed for the extraction of essential oil or
Volatile oils from plant and the common ones are:
i) Hydro-distillation method, e. g. turpentine oil;
ii) Expression method, e.g. orange oil;
iii) Effleurage method and
iv) Solvent extraction method, e. g. rose oil,
All these processes consist of removing the odoriferous substances aromatic plant.
Hydro-distillation methods:
This method not only produces the best yields but also enables the operator to attain the end sought most cheaply and with the simplest apparatus. Further, larger quantities of oil can thus be produces without much human labour.
Hydro-distillation methods are three types:
i) Water distillation
ii) Water and steam and distillation
iii) Steam distillation
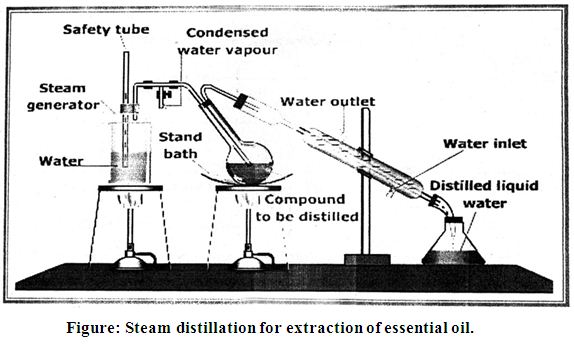
The majority of essential oils have always been obtained by steam distillation or by hydro distillation or by solvent extractor. Essential oil or volatile oils are the mixture composed of volatile substances like hydrocarbon, ether, alcohols, esters etc Distillation may be defined as the separation of the components of mixture of two or more liquids by virtue of the difference in their vapor pressure occurred when steam depresses the vapor pressure of essential oil. So extraction takes place when the mixed vapor was condensed, then system of water & essential oil forms a two phases liquid; therefore this type of distillation is of primary importance to the essential oil producer.
Principle of steam distillation:
A liquid boils when its vapor pressure is equal to the atmospheric- pressure .In steam distillation, a mixture of water and an organic liquid is heated. The mixture boils when the combined vapor pressure of water (p1) and that of the organic liquid (p2) is equal to the atmospheric Pressure (P) i.e.P = p1 + p2
The oils were isolated by hydro-distillation using Clevenger’s apparatus for 4 hrs and then dried over anhydrous sodium sulphate.
Steam distillation for extraction of essential oil of Ginger:
The essential oils were obtained by steam distillation from the fresh ginger rhizome materials. For this purposes it were collected from the different region of Bangladesh such as Chittagong ginger and China ginger.
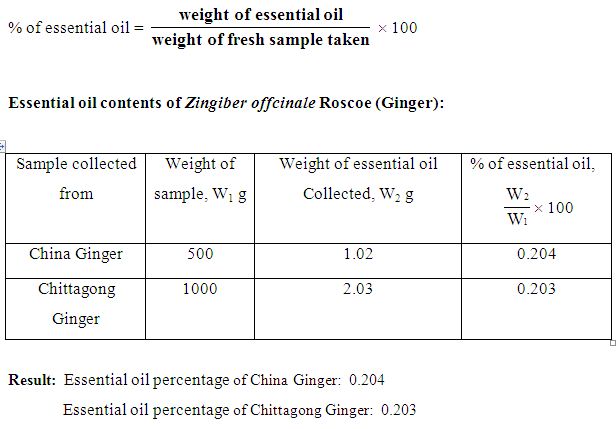
After blending definite amount (500g) of sample (dirt free fresh ginger rhizome) were taken in the distillation flask (Clevenger’s apparatus). Then water was added in the flask two third of its volume. The flask with crushed sample and water heated by means of electric heating mental for 4 hours. Volatile substances of Ada and steam that produce in the flask were condensed by water condenser. The essential oil was lighter than water and separated. The steam distilled essential oil layer, which was collected over water, was extracted and washed with analytical grade ether or chloroform. The ether extract of the oil was dried over anhydrous Na2SO4 the filtered. It was collected in vial. The ether or chloroform was removed in vacuum environment and weighted refrigerated at 40C. The percentage of the essential oil content was calculated by the following formula:
Procedure for determination of Sp. Gravity (Pycnometer method):
The pycnometer was cleaned by filling it with a saturated solution of chromium trioxide in Conc. sulphuric acid (H2SO4) and allowed it to stand for at least three hours. The solution was removed from the pycnometer and rinsed with distilled water. Then it was dried completely and it was permitted to stand for 30 minutes.
Then the empty pycnometer was weighed accurately by an electronic balance and it was filled with distilled water up to the mark and weighed.
The pycnorneter was then dried and filled with sample oil to the previous mark and
Weighed again.The specific gravity of the essential oil was calculated by the following formula.
Determination of Refractive Index [ht°C]:
The refractive index [h] of a substance is the ratio of the speed of light in a vacuum to
the speed of light in the substance. The index of refraction of oils is characteristics
within certain limits for each kind of oil. It is related to the degree of saturation but is affected by other factors such as free fatty acid content oxidation and heat treatment.
This method is applicable to all normal oils and liquid fats.
The following general rules can be outlined regarding the index of refraction:
- It increases together with the increase of molecular weight of the fatty acid.
- It increases together with the increase of number of existing double bonds.
- For any simple glyceride it is higher than that of the corresponding fatty acids or oil.
- It increases when temperature increases by about 0.00038/°C.
Principle:
The refractive index [h] of a substance is defined as the ratio of the velocity of light in
vacuum or air to that in the substance:
When a ray of light passes from air into a liquid it’s
direction is changed. This change of direction is called refraction.
The refractive index of a solid and liquid are conveniently determined when ray of monochromatic light passes from a less dense to a denser medium; it is bent or refracted towards the normal. Thus in figure if,(I) is the less dense and (II) is the more dense medium, a ray of light passing from (I) to (II) will be bent so that the angle of refraction ‘r’ will be less than the angle of incidence ‘i’ ( i > r).
And according to the law of refraction, the relation between these two angles will be
Where ‘n’ is the index of refraction of the less dense, and ‘N’ the index of refraction of the more dense medium. As the angle ‘i’ increases the angle ‘r’ also increases, and reaches its maximum volume ‘r’ when ‘i’ becomes equal to a right angle. That is, when the incident light is horizontal; since i =900 then. Sin i = Sin 900=1.
The above equation becomes, Or, Sin r =
If i > 900 the ray is totally internally reflected in total internal reflection occurred in the medium concerned.
Procedure for determination of refractive index:
The instrument was placed in such a position that diffused day light or some form of artificial light (such as sodium light) can readily be obtained for illumination. The prism was cleaned carefully with alcohol and then with ether. The double prism was opened by means of the screw head and few drops of fatty oil placed on the prism. For solid fat the temperature should be suitably adjusted by circulating hot water. The prism was closed firmly by tightening the screw head. The instrument was allowed stand for few minutes before the reading was noted so that the sample and the instrument attained the same temperature. The alidade backward or forward was moved until the field of vision was divided into a light and dark portion by a line and this line appeared in the form of a band of colour. The screw head was rotated in such a way that the colour band disappeared while a sharp colourless line was obtained.The reading was taken from the scale and second reading was taken a few minutes later to assure that temperature equilibrium was attained.
Table: Refractive index [h]t° of Essential oil:
Sample collected from | Refractive index [h]t° of Essential oil at 300C |
China Ginger | 1.4873 |
Chittagong Ginger | 1.4875 |
Determination of Optical rotation, [a]tD:
The emerging beam of light having oscillation in a single plane is said to be plane polarized. When plane-polarized light is passed through certain solution of optical active Substance, the plane of polarized light is rotated; the field of view appears alternately light and dark. A compound that can rotate the plane of polarized light is called optically active. This property of a compound is called optical activity. The prism, of which the light is polarized, is called the polarizer and the second prism by which the light is examined is called the analyzer. In order to obtain darkness, the analyzer has to be turned to the right, i.e. clock wise, the optically active substance is said to be dextrorotatory, and levorotatory when the analyzer must be turned to the left. By convention, rotation to the left is given a minus sign (-) and rotation to the right is given a plus sign (+). One complete rotation of the prism through 3600, there are two positions of the unalyzer, 1800 apart, at which the field is dark, and similarly, two positions at which there is a maximum of brightness. Lndetermining the sign of the activity of a substance, one takes the direction in which the rotation required to give extinction is less than 90°.
The angle of rotation depends on:
- The nature of substance
In order to obtain a measure of the rotator power of a substance, these factors must be taken into account and one then obtains the specific rotation. Most essential oil when placed in a beam of polarized light rotates the plane of polarized light to the right (dextrotatory) or to the left (laevorotatory). The extent of the optical activity of oil is determined by a polarimeter.
Specific Rotation:
Specific rotation is defined as the angle of rotation produced by a liquid which in the volume of l ml contains 1g of active substance, when the length of the column through which the light passes is l dcm. The specific rotation is represented by (o) the observed angle of rotation being represented simply by ‘a’ conventionally a specific rotation is
reported as [a]tD, where ‘t’ stands for temperature and for ‘D’ line of sodium used for determination.When the active substance is examined in solution, the concentration must be taken into account, is accordance with the expressions:
Where,
[a] = Specific rotation in degree
a = Observed angle of rotation in degrees.
l = Length of the sample solution in decimeters
c = concentration of the sample solution in g/ml
or. The number of grams of active substance in l00 ml of solution,
Instrument model: WXG – 4, Made in: China
Procedure for determination of optical rotation:
About 0.5g oil or fat materials was dissolved in 50 ml chloroform to prepare 1% solution. The solution was put to a polarimeter tube so that no bubble remained in it. The light source was so adjusted that when the analyzer is at zero position the field appears bright. The analyzer was then rotated until the field was uniformly bright. This was the zero point of the instrument. This was determined by approaching from either side several times and the means of the reading were taken.
The tube was then emptied cleaned and tilled with chloroform. Another set of reading with the chloroform was taken. The difference between the two readings gave the optical rotation.
Optical rotation of fatty oil of Zingiber officinale Roscoe (Ginger):
| Sample collected from
| Optical rotation of essential oil of at 260C [a]26D |
| China Ginger | -39.40C |
| Chittagong Ginger | -38.20C |
Determination of solubility of Zingiber officinale Roscoe (Ginger) essential oil:
Procedure for determination of solubility:
Introduce Exactly 1 cc of the oil into a test-tube, and was added slowly in small portions of alcohol (10 drops=1volume) of proper strength. The cylinder was shaking thoroughly after each addition. When a clear solution is first obtained, recorded the strength and the number of volumes of alcohol required was recorded. The addition of alcohol until 10cc has been added. If opalescent or cloudiness occurs during these subsequent additions of alcohol, record the point at which this phenomenon occurs. In the event that a clear solution is not obtained at any point during the addition of the alcohol, the determination was repeated, using an alcohol of higher strength.
Determination of Solubility in alcohol of different strength:
Solubility in alcohol of different strength in Ginger essential oil was observed below:
01. Test with 70% alcohol:
On addition of 0.2 ml alcohol the oil and alcohol layer was distinct and the oil remained at the bottom.
On further addition of 9.8 ml of alcohol a great number of oil fragments was found to float on the mixture.On further addition of 10 ml alcohol the amount of oil fragments were decreased but not went into solution completely.
02. Test with 80% alcohol:
On addition of 0.4 ml of alcohol on few drops of oils, the oil and alcohol
layer was distinct and the oil remained at the bottom.
On further addition of 3.6 ml of alcohol a great number of oil fragments were
found and floated on the mixture.
On further addition of 5.0 ml of alcohol, then clear solution was obtained.
03. Test with 90% alcohol:
Addition of 0.1 ml alcohol to the oil clearly soluble on further addition of alcohol.
04. Test with 95% alcohol:
0.1 ml of 95% alcohol was added, the oils and a clear solution were formed. On further addition of alcohol, no turbidity was observed but it is not always clear.
Colour Test:
The characteristic colour of most oils is predominantly a mixture of yellow and red it is due primarily to the presence of pigments of a carotenoid type. Other colour like blue, green and brown are also not uncommon.
Table : Colour observation test for essential oil of Zingiber officinale Roscoe (Ginger):
Sample collected from | Colour |
China Ginger | Golden yellow |
Chittagong Ginger | Golden yellow |
Appearance (25-300C):
Fatty oil of Zingiber officinale Roscoe (Ginger):
- Homogeneous
- Golden yellow transparent liquid and
- Lighter than water.
- Bitter in taste
- Spicy odor
– at room temperature (30°C)
Determination of Chemical Properties of Essential oil:
Determination of Acid Value
Most essential oil contain only small amount of free acids. Acid value denotes the number of mg of potassium hydroxide (KOH) needed to neutralize the free acids in 1 g of fat or oil. Acid value indicates the proportion of free fatty acid in the oil or fat. The free fatty acid is produced by the hydrolytic decomposition of the oil. The low acid value is an indication of freshness of the oil and order the materials higher is the amount of the free acid.
Reagents:
- 95 % aqueous neutral ethanol.
- 0.1N (aq) NaOH solution
- 0. 1N (aq) Na2CO3 solution
- 0.1N HC1
- 0.1%Methyl orange indicator
- 1% phenolphthalein solution.
Procedure for the Normality determination of 0.1N NaOH solution:
a) Preparation of 0.1 N Na2CO3Solution,
b) Preparation of 0. 1 N HC1 solution,
c) Standardization of HC1 by primary standard Na2CO3 solution,
d) Preparation of 0.1 N NaOH Solution,
e) Standardization of NaOH by HC1,
Determination of Ester value:
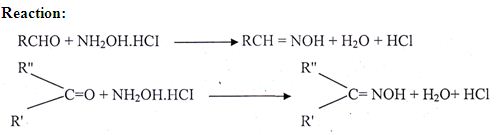
The esters value is a relative measure of the amount of ester present. The ester in the essential oil is expressed by the ester number. It is defined as the number of milligrams of KOH, required to saponify the ester present in 1 gm of the oil.
The determination of the ester value is of great importance in the evaluation of many essential oil. The processes of saponification of ester may be represented by the following reaction.
RCOOR+ NaOH ® RCOONa + R’OH
Where R and R’ may be an aliphatic, aromatic or alicyclic radical. ‘R’may also is a hydrogen atom.
Reagents:
a) 95% alcohol.
b) 0.1 (N) aqueous KOH solution.
c) 0.5 (N) alcoholic KOH solution.
d) 0.5 (N) aqueous HC1 solution.
e) 1% alcoholic phenolphthalein indicator,
f) 0.5 N Na2CO3 solution
Standardization of HC1 by 0.5 N Na2CO3 solutions:
10 ml of 0.5 N Na2CO3 solutions was taken in a conical flask with the help of pipette. 50 ml distilled water and few drops of methyl orange added to the solution and the solution became yellowish colour. 0.5N HC1 was taken in a burette. HC1 added drop wise into Na2CO3 solution. At the end point of titration the colour of the solution became rosy colour. Titration was carried out exactly three times. The results are tabulated as follows.
Reaction: Na2CO3 + 2HC1 = 2NaCl + CO2 + H2O
The results are tabulated as follows:
Table: Titration Data (Standardization of HCI by 0.5 N Na2CO3 solution):
Observe No. | Na2CO3 solution (ml) | Burette reading Volume of HCI solution (ml) | |||
| Initial reading | Final reading | Difference | Average volume | ||
1. | 10 | 0.0 | 9.1 | 9.1 |
9.1 |
2. | 10 | 0.0 | 9.1 | 9.1 | |
3. | 10 | 0.0 | 9.1 | 9.1 | |
Calculation:
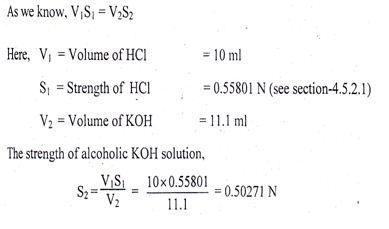
As we know, V1S1 = V2S2
Here, V1 = Volume of Na2CO3 solution = 10 ml
S1 = Strength of Na2 CO3 solution = 0.5050 N
V2 = Volume of HC1 solution = 9.1 ml
Procedure for determination of ester value:
1.5263 g of the oil was weighed accurately into a 100 ml alkali resistant saponification flask. 5 ml of neutral 95% ethanol and 3 drops of 1% alcoholic phenolphthalein were added to the saponification flask. The free acids were neutralized with standard 0.1 N aqueous KOH solutions. Then 10 ml of 0.5 N alcoholic KOH was added which was measured accurately from a pipette. An air cooled condenser of 1m in length and 1 cm in diameter was attached to the flask.
The contents of the flask were refluxed for 1 hr on a steam bath. The flask was removed and permitted to cool at room temperature. The excess alkali was titrated with standard 0.5 N aqueous solution of HCl.
In order to determine the amount of alkali consumed, a blank determination was carried out at the same condition in different experiments with different amounts of oil.The difference in the amounts of acid used in titrating the actual determination and the blank gave the amount of alkali used for the saponification of the esters.
Calculation method:
The ester value of the oil was found calculated by the following formula:
Where
B = Blank determination (Volume of 0.5 N HCl in ml)
S = Sample determination (Volume of 0.5 N HCl in ml)
Shc1= Strength of HC1
W= Weight of the sample taken in gm.
Table -: (Blank Titration with 0.5 N alcoholic HC1 solution)
Observe No. | Burette reading ,Volume of 0.5 N HC1 solution (ml) | |||
| Initial reading | Final reading | Difference | Average volume | |
1 | 0.0 | 9.2 | 9.2 | 9.2 |
2 | 0.0 | 9.2 | 9.2 | |
3 | 0.0 | 9.2 | 9.2 | |
Result: The blank determination of 0.5 N HC1, B = 9.2ml
Essential Oil Analyses by Gas Chromatography and Mass Spectrometer (GC-MS)
Gas chromato graph-Mass Spectrometer (GS-MS) is an integrated composite analysis instrument combine GC, which is able to separate and quantify the components, with MS which is also able to identify each component. It has made remarkable progress and is commonly used in the fields of organic chemistry, medical science, pharmacy etc. It has come into use and also for analysis in the environmental field. GC-MS 2010 plus is a high performance quadruple mass spectrometer. Two systems, column and direct inlet are available with this instrument to enter the samples into the ion box. It has two methods for sample analysis such as electron impact ionization (El) and chemical ionization (CI). Conventional El method is thought of as a hard ionization and chemical ionization as thought of as a soft ionization. Negative chemical ionization (NCI) is an especially sensitive method for substances with high electro negativity, such as halogenated compounds. A library having 1, 07,000 spectra of organic and organometalic compounds has been integrated with PC to identify the selected compounds. Name, molecular weight, formula and structure of compounds can be established by matching and comparing the mass fragment patterns of their mass spectrums with those of the library enlisted compounds.
Apparatus and Operating conditions for GC-MS analysis:
a) Gas chromatograph: The analysis was carried out by GC-MS electron impact
ionization (El) method on GC-17A gas chromatograph coupled to a GC-MS-2010 plus. Mass-Spectrometer.
b) Name of column: RTS-5MS, Dimiter 30m, Lenth 0.25mm
c) Temperature of column: Initial Temperature 40°, held at 40°C for 2min,
d) Injector temperature: Injection port temperature 220°C,helding time 5 min
e) Column Packing: Column packing was done with 10% diethylene glycol
succinate on 100 – 120 mesh diatomic CAW
f) Splitting: Samples were injected by splitting with the split ratio 10.
i) Carrier gas: Helium gas at constant pressure 90 kPa.
g) Sample dissolved: In chloroform.
h) Range of linear temperature increase: 100C per min.
GC-MS analyses of essential oil of of Zingiber officinale Roscoe Bangladeshi Ginger and China Ginger:
The essential oils of Zingiber officinale Roscoe (Ginger) were analysis by Electron Impact lonization (El) method on GC-17A Gas Chromatograph, coupled to a GC-MS 2010 plus Mass Spectrometer; fused silica capillary column (30m x 0.25 mm). Column temperature of 40°C (held 2 min) was maintained with carrier gas helium at a constant pressure of 90 kPa Samples were injected by splitting with the split ratio 10. Essential oil sample was dissolved in chloroform.
The GC-MS report of the essential oil was given in Table-25&26 The GC-MS chromatogram of the essential oil were shown in Figure-1,2,3,4, 5,6,7,8,9,10&11 and the spectrum for compound information of GC-MS peaks collected from GC-MS were shown to be Figure-1,2,3,4,5,6,7,8,9,10&11
Procedure GC-MS analyses of essential oil Zingiber officinale Roscoe: Essential oil was diluted to 7% by chloroform. An inert carries gas (i.e. nitrogen) was introduce, from a large gas cylinder through the injection part, the column and the detector. The flow rate of the carrier gas was adjusted to insure reproducible retention times and to minimize detector drift. The sample was then injected by a micro syringe through a heated injection part when it was vaporized and carried into the column.
The long tube of the column was tightly packed with solid particles. The solid support was uniformly covered with a thin film of a high boiling liquid (the stationary phase). The mobile and stationary phases were then partition by the sample and it was separated into its individual components.
The carrier gas and sample component was then emerging from the column and passed through a detector. The amount of each component was measured as concentration by this device and generates a signal which was registered electrically. This signal passed to a recorder.
The standard rcferance sample were analysed by the same procedure.
Identification: The retention distance of essential oil and other reference samples were measured. The essential oil peaks were compared with peaks of reference sample by overlapping the chromatogram.
Fatty Oil
Fats and fatty acid and its relation to the plant materials:
A non volatile oil composed of fatty acids, usually of animal or vegetable origin is called fatty oil or fixed oil.
Nature has endowed the world with a variety of important oil bearing plants and animals. Vegetable oils and fats are essential items of human consumption either as such or in refined or hydrogenated form.The fatty acids that occur in nature usually have straight chains and usually contain even number of carbon atoms. They are esters of glycerol. These compounds are known as glycerol esters. They are either oily liquids or waxy solids and contain the element carbon, hydrogen and oxygen only individual fat is usually a mixture of esters of glycerol and two or three different fatty acids. Fatty acid is also found free and in lore molecular complexes in protein.
Every fat molecule has essentially four parts: The core of the molecule is glycerol, a three carbon compound that is related to the alcohol. Three fatty acids combined with the glycerol molecule to form a fat. The nature of a fat depends on what kinds of fatty acids are linked to the glycerol core, the number of carbon atoms of the fatty acids, and the degree of saturation or unsaturation of the fatty acids.
A fat molecule may be composed of three identical fatty acids, three different ones, or a combination of two alike and one different, when all of the fatty acids in a fat molecule are identical. The molecule is called a simple triglyceride; a molecule with different fatty acids is called a mixed triglyceride. The mixed triglycerides are found in both animal and vegetable foods.Fatty acids are long chain carboxylic acids, it contain only one carboxylic group and thus monobasic. They fall roughly into three groups; saturated, unsaturated and those, which contain branches or rings. Although numerous fatty acids are now known in plants, the palmitic acid (C-16) is the major saturated acid in leaf lipids and also occur in varying quantities in some seeds oils. Stearic acid (C-18) is the major saturated acid in seed fats of a number of plant families’ l0; unsaturated acids mainly C-16 and C-lg are widespread in both leaf and seed oil. A number of rare fatty acids (e.g. erucic and sterculic acid) are found in seed oils of a few plants. The plant glycerides have a relatively higher proportion of the more unsaturated acids.
Although alkaloids, terpenoids, steroids, f1avones and their glycosides, phenols and phenolic acids constitute a major portion of non – carbohydrate materials of a plant, fatty acids are always present in varying amounts in all plant materials. They occur in plants primarily in bonds form as fats or Lipids. These lipids comprise upto 70% of the dry weight in leaves in higher plants, about l-5% in stems of green plants. These are important as membrane constituent in the chloroplasts and mitochondria. These substances also occur in considerable amounts in the seeds of fruits of a number of plants and act as a storage form of energy to be used during germination. These fatty materials influence the handling of the plant tissues as well as any chemical treatment done in it.Seed oils from plants such as olive, palm, coconut etc. are exploited commercially and used as edible oils. For soap manufacture and in the paint industry therefore the study of fatty acids.The major constituent of all fatty matters is important. A fatty acid is a carboxylic acid often with a long unbranched aliphatic, tail (chain), witch is either saturated or unsaturated. Fatty acids derived from natural fats and oils may be assumed to have at least 8 carbon atoms, e.g. caprylic acid (octanoic acid). Most of the natural fatty acids have an even number of carbon atoms, because their biosynthesis involves acetyl-Coenzyme carrying a two-carbon-atom group. In nature they are found as glyceryl esters.
Classification of fatty acids:
Fatty acids are two types on the basis of the nature of the bonds in the acid molecule.
They are –
1) Unsaturated fatty acid
2) Saturated fatty acid
Examples:
Some examples of saturated and unsaturated fatty acids are given below.
1) Unsaturated fatty acid:
Name of the acid | Chemical formula |
| Myristoleic acid: Palmitoleic acid: Oleic acid: Linoleic acid: Alpha-linolenic acid: Erucic acid: | CH3(CH2)3CH=CH(CH2)7COOH CH3(CH2)5CH=CH(CH2)7COOH CH3(CH2)7CH= CH(CH2)7COOH CH3(CH2)4CH=CHCH2CH=CH(CH2)7COOH CH3CH2CH=CHCH2CH=CHCH2C=CH(CH2)7COOH CH3(CH2)7CH= CH(CH2)11COOH |
2) Saturated fatty acid:
| Name of the acid | Chemical formula |
| Butyric Caproic Caprylic Capric Lauric. Myristic Pahnitic Stearic Arachidic Behenic Lignoceric
| CH(CH2)2COOH CH(CH2)4COOH CH(CH2)6COOH CH(CH2)8COOH CH(CH2)10COOH CH(CH2)12COOH CH(CH2)14COOH CH(CH2)16COOH CH(CH2)18COOH CH(CH2)20COOH CH(CH2)22COOH
|
Necessary Fatty Acids:
The human body can produce all but two of the fatty acids it needs. These two are called essential fatty acid. They are linoleic acid (LA) and alpha-linolenic acid (LNA) and are widely distributed in plant oils. In addition, fish oils contain the longer chain omega-3 fatty acids ecosapentaenoic acid (EPA) and docosahexaeiroic acid (DHA). Essential fatty acids are poly unsaturated fatty acids and are the parent compounds of the omega-6 Omega-3 fatty acid series respectively.
Extraction of fatty oil:
The fatty oil sample was extracted by using extraction method by using 40- 600 C. pet ether as solvent from crushed (essential oil and moisture free) sample in a glass Sox let apparatus as following.
Soxh let extraction for collection of fatty oil.
The residue obtained after steam distillation of sample taken in a thimble, which was prepared by filter paper. Then the definite amount of moisture and essential oil free sample in thimble was placed in the Sox let apparatus unit and extraction was carried out with 40°- 60° c. petroleum ether for 30 hrs. in a water bath at 80°C-90°C.
Purification of fatty oil:
The fatty oil in the solvent obtained from the sox let unit was filtered to remove impure materials. The filtrate containing the fatty oil was distilled at low temperature on the water both. Thus the solvent was removed from the mixture. The trace amount of solvent remained in the fatty oil was eliminated by using high vacuum.Then dried in an oven at 80°C. And cooled in desiccators and weighed. By this process the purified fatty oil was obtained for further characterization.
Calculation method:
Ihe percentage of the fatty oil content was calculated by the following formula:
The percentage of the fatty oil obtained = x 100
Weight of sample taken= W1 g
Weight of fatty oil extractracted= W2 g
% of fatty oil= x 100
Table : Fatty oil contents of Zingiber officinale Roscoe (Ginger):
Sample collected from | Weight of sample, (W1) g
| Weight of fatty oil collected, (W2) g | % of fatty oil, ´ 100 |
China Ginger | 114.056 | 0.99 | 0.867 |
Chittagong Ginger | 134.82 | 2.68 | 1.98 |
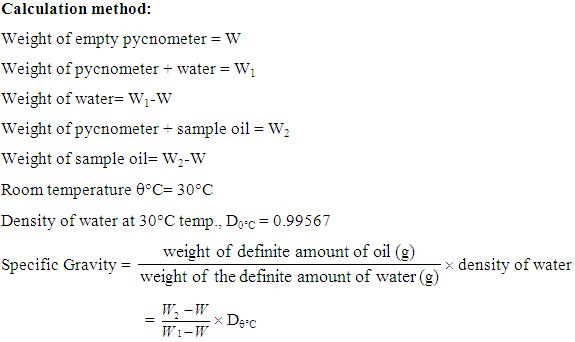
Procedure for determination of Sp. Gravity (Pycnometer method):
The pycnometer was cleaned by filling it with a saturated solution of chromium trioxide in Conc. sulphuric acid (H2SO4) and allowed it to stand for at least three hours. The solution was removed from the pycnometer and rinsed with distilled water. Then it was dried completely and it was permitted to stand for 30 minutes.
Then the empty pycnometer was weighed accurately by an electronic balance and it was filled with distilled water up to the mark and weighed. The pycnorneter was then dried and filled with sample oil to the previous mark and weighed again.
The specific gravity of the essential oil was calculated by the following formula.
Calculation method:
Weight of empty pycnometer= W
Weight of pycnometer + water= W1
Weight of water= W1-W
Weight of pycnometer + sample oil = W2
Weight of sample oil= W2-W
Room temperature q°C= 30°C
Density of water at 30°C temp., D0°C = 0.99567
Specific Gravity = ´ density of water
= ´ Dq°C
Table : Specific Gravity of fatty oil:
| ||||||||||||
|
Determination of Refractive Index [ht°C]:
The refractive index [h] of a substance is the ratio of the speed of light in a vacuum to
the speed of light in the substance. The index of refraction of oils is characteristics
within certain limits for each kind of oil. It is related to the degree of saturation but is affected by other factors such as free fatty acid content oxidation and heat treatment.This method is applicable to all normal oils and liquid fats.
The following general rules can be outlined regarding the index of refraction:
- It increases together with the increase of molecular weight of the fatty acid.
- It increases together with the increase of number of existing double bonds.
- For any simple glyceride it is higher than that of the corresponding fatty acids or oil.
- It increases when temperature increases by about 0.00038/°C.
Principle:
The refractive index [h] of a substance is defined as the ratio of the velocity of light in
vacuum or air to that in the substance:
When a ray of light passes from air into a liquid it’s
direction is changed. This change of direction is called refraction.
The refractive index of a solid and liquid is conveniently determined when ray of monochromatic light passes from a less dense to a denser medium; it is bent or refracted towards the normal. Thus in figure if,(I) is the less dense and (II) the more dense medium, a ray of light passing from (I) to (II) will be bent so that the angle of refraction ‘r’ will be less than the angle of incidence ‘i’ ( i > r).
And according to the law of refraction, the relation between these two angles will be
such that: =
Where ‘n’ is the index of refraction of the less dense, and ‘N’ the index of refraction of the more dense medium. As the angle ‘i’ increases the angle ‘r’ also increases, and reaches its maximum volume ‘r’ when ‘i’ becomes equal to a right angle. That is, when the incident light is horizontal; since i =900 then. Sin i = Sin 900=1.
The above equation becomes, Or, Sin r =
If i > 900 the ray is totally internally reflected in total internal reflection occurred in the medium concerned.
Procedure for determination of refractive index:
The instrument was placed in such a position that diffused day light or some form of artificial light (such as sodium light) can readily be obtained for illumination. The prism was cleaned carefully with alcohol and then with ether. The double prism was opened by means of the screw head and few drops of fatty oil placed on the prism. For solid fat the temperature should be suitably adjusted by circulating hot water. The prism was closed firmly by tightening the screw head. The instrument was allowed stand for few minutes before the reading was noted so that the sample and the instrument attained the same temperature. The alidade backward or forward was moved until the field of vision was divided into a light and dark portion by a line and this line appeared in the form of a band of colour. The screw head was rotated in such way that the colour band disappeared while a sharp colourless line was obtained.
The reading was taken from the scale a second reading was taken a few minutes later to assure that temperature equilibrium was attained.
Table: Refractive index [h]t° of fatty oil:
Sample collected from | Refractive index [h]t° of Essential oil at 300C |
China Ginger | 1.448 |
Chittagong Ginger | 1.4525 |
Determination of Optical rotation, [a]tD:
The emerging beam of light having oscillation in a single plane is said to be plane polarized. When plane-polarized light is passed through certain solution of optical active substance, the plane of polarized light is rotated; the field of view appears alternately light and dark. A compound that can rotate the plane of polarized light is called optically active. This property of a compound is called optical activity. The prism, of which the light is polarized, is called the polarizer and the second prism by which the light is examined is called the analyzer. In order to obtain darkness, the
analyzer has to be turned to the right, i.e. clock wise, the optically active substance is said to be dextrorotatory, and levorotatory when the analyzer must be turned to the left. By convention, rotation to the left is given a minus sign (-) and rotation to the right is given a plus sign (+). One complete rotation of the prism through 3600, there are two positions of the unalyzer, 1800 apart, at which the field is dark, and similarly, two positions at which there is a maximum of brightness. lndetermining the sign of the activity of a substance, one takes the direction in which the rotation required to give extinction is less than 90°.
The angle of rotation depends on:
- The nature of substance
In order to obtain a measure of the rotator power of a substance, these factors must be taken into account and one then obtains the specific rotation.
Most essential oil when placed in a beam of polarized light rotates the plane of polarized light to the right (dextrotatory) or to the left (laevorotatory). The extent of the optical activity of oil is determined by a polarimeter.
Specific Rotation:
Specific rotation is defined as the angle of rotation produced by a liquid which in the volume of l ml contains 1g of active substance, when the length of the column through which the light passes is l dcm. The specific rotation is represented by (o) the observed angle of rotation being represented simply by ‘a’ conventionally a specific rotation is reported as [a]tD, where ‘t’ stands for temperature and for ‘D’ line of sodium used for determination.When the active substance is examined in solution, the concentration must be taken into account, is accordance with the expressions:
Where,
[a] = Specific rotation in degree
a = Observed angle of rotation in degrees.
l = Length of the sample solution in decimeters
c = concentration of the sample solution in g/ml
or. The number of grams of active substance in l00 ml of solution,
Procedure for determination of optical rotation:
About 0.5g oil or fat materials was dissolved in 50 ml chloroform to prepare 1% solution. The solution was put to a polarimeter tube so that no bubble remained in it. The light source was so adjusted that when the analyzer is at zero position the field appears bright. The analyzer was then rotated until the field was uniformly bright. This was the zero point of the instrument. This was determined by approaching from either side several times and the means of the reading were taken.
The tube was then emptied cleaned and tilled with chloroform. Another set of reading with the chloroform was taken. The difference between the two readings gave the optical rotation.
By this procedure, the value of optical rotation of sample fatty oil was found:
Table: Optical rotation of fatty oil of Zingiber officinale Roscoe (Ginger):
| Sample collected from
| Optical rotation of essential oil of at 260C [a]26D |
| China Ginger | -39.40C |
| Chittagong Ginger | -38.20C |
Determination of solubility in different solvents:
As several industrial processes are based on the property that fatty acids and fats have of dissolving property in some solvents, it is important to know how these oily substances to behave when they come into contact with these solvents. The most widely used solvents are hexane, isopropyl alcohol, acetone and water.
Procedure for determination of solubility:
Introduce Exactly 1 cc of the oil into a test-tube, and was added slowly in small portions of alcohol (10 drops=1volume) of proper strength. The cylinder was shaking thoroughly after each addition. When a clear solution is first obtained, recorded the strength and the number of volumes of alcohol required was recorded. The addition of alcohol until 10cc has been added. If opalescent or cloudiness occurs during these subsequent additions of alcohol, record the point at which this phenomenon occurs. In the event that a clear solution is not obtained at any point during the addition of the alcohol, the determination was repeated, using an alcohol of higher strength.
Tabl: Solubility test of fatty oil of Zingiber officinale Roscoe (Ginger) in different solvent:
Solubility In | Sample collected from | |
China Ginger | Chittagong Ginger | |
Alcohol | Soluble | Soluble |
Distilled water | Insoluble | Insoluble |
Chloroform | Soluble | Soluble |
CCl4 | Soluble | Soluble |
Pet-ether | Soluble | Soluble |
Diethyle ether | Soluble | Soluble |
n-Hexane | Insoluble | Insoluble |
The solubility test of fatty oil was carried out in a test of solvent like alcohol, Chloroform, CCl4, Pet-ether, n-Hexane, Diethyl ether of the fatty oil was determined at the same procedure.
Remarks:
- Insoluable in n-Hexane and Distilled water at any volume.
- Soluable in Alcohol, Chloroform, CCl4, Pet-ether, , Diethyle ether at any volume.
Colour Test:
The characteristic colour of most oils is predominantly a mixture of yellow and red it is due primarily to the presence of pigments of a carotenoid type. Other colour like blue, green and brown are also not uncommon.
Table: Colour observation test for fatty oil of Zingiber officinale Roscoe (Ginger):
Sample collected from | Colour |
China Ginger | Dark brown |
Chittagong Ginger | Dark brown |
Appearance at room temperature:
Fatty oil of Zingiber officinale Roscoe (Ginger):
- Homogeneous
- Dark brown transparent liquid and
- Lighter than water.
- Bitter in taste
- Spicy odor
– at room temperature (30°C)
Determination of Chemical Properties of Fatty oil:
Determination of Acid Value
Most fatty oil contain only small amount of free acids. Acid value denotes the number of mg of potassium hydroxide (KOH) needed to neutralize the free acids in 1 gm of fat or oil. Acid value indicates the proportion of free fatty acid in the oil or fat. The free fatty acid is produced by the hydrolytic decomposition of the oil. The low acid value is an indication of freshness of the oil and order the materials higher is the amount of the free acid.
Determination of Ester value:
The esters value is a relative measure of the amount of ester present. The ester in the fatty oil is expressed by the ester number. It is defined as the number of milligrams of KOH, required to saponify the ester present in 1 gm of the oil.
The determination of the ester value is of great importance in the evaluation of many fatty oils. The processes of saponification of ester may be represented by the following reaction.
RCOOR+ NaOH ® RCOONa + R’OH
Where R and R’ may be an aliphatic, aromatic or alicyclic radical. ‘R’may also is a hydrogen atom.
Reagents:
a) 95% alcohol.
b) 0.1 (N) aqueous KOH solution.
c) 0.5 (N) alcoholic KOH solution.
d) 0.5 (N) aqueous HC1 solution.
e) 1% alcoholic phenolphthalein indicator,
f) 0.5 N Na2CO3 solution
Standardization of HC1 by 0.5 N Na2CO3 solution:
10 ml of 0.5 N Na2CO3 solutions was taken in a conical flask with the help of pipette. 50 ml distilled water and few drops of methyl orange added to the solution and the solution became yellowish colour. 0.5N HC1 was taken in a burette. HC1 added drop wise into Na2CO3 solution. At the end point of titration the colour of the solution became rosy colour. Titration was carried out exactly three times. The results are tabulated as follows.
Reaction: Na2CO3 + 2HC1 = 2NaCl + CO2 + H2O
The results are tabulated as follows:
Table-: Titration Data (Standardization of HCI by 0.5 N Na2CO3 solution):
Observe, No. | Na2CO3 solution (ml) | Burette reading , Volume of HCI solution (ml) | |||
| Initial reading | Final reading | Difference | Average volume | ||
1 | 10 | 0.0 | 48.4 | 48.4 | 48.2 |
2 | 10 | 0.0 | 48.2 | 48.2 | |
3 | 10 | 0.0 | 48.1 | 48.1 | |
Calculation:
As we know, V1S1 = V2S2
Here, V1 = Volume of Na2CO3 solution = 10 ml
S1 = Strength of Na2 CO3 solution = 0.5050 N
V2 = Volume of HC1 solution = 48.2 ml
The strength of HC1 solution,
S2 =
So the strength of HC1 acid solution was 0.10 N
Procedure for determination of Zingiber officinale Roscoe (Ginger) ester value:
1.5263 g of the oil was weighed accurately into a 100 ml alkali resistant saponification flask. 5 ml of neutral 95% ethanol and 3 drops of 1% alcoholic phenolphthalein were added to the saponification flask. The free acids were neutralized with standard 0.1 N aqueous KOH solutions. Then 10 ml of 0.5 N alcoholic KOH was added which was measured accurately from a pipette. An air cooled condenser of 1 m in length and 1 cm in diameter was attached to the flask. The contents of the flask were refluxed for 1 hr on a steam bath. The flask was removed and permitted to cool at room temperature. The excess alkali was titrated with standard 0.5 N aqueous solution of HCl. In order to determine the amount of alkali consumed, a blank determination was carried out at the same condition in different experiments with different amounts of oil. The difference in the amounts of acid used in titrating the actual determination and the blank gave the amount of alkali used for the saponification of the esters.
Calculation method:
The ester value of the oil was found calculated by the following formula:
Ester value of fatty oil =
Where,
B = Blank determination (Volume of 0.5 N HC1 in ml)
S = Sample determination (Volume of 0.5 N HC1 in ml)
SHCl =Strength of HC1
W= Weight of the sample taken in gm.
Table: (Blank Titration with 0.5 N alcoholic HC1 solution)
Observe No. | Burette reading ,Volume of 0.5 N HC1 solution (ml) | |||
| Initial reading | Final reading | Difference | Average volume | |
1 | 0.0 | 58.9 | 58.9 | 58.93 |
2 | 0.0 | 59.0 | 59.0 | |
3 | 0.0 | 58.8 | 58.8 | |
Result: The blank determination of 0.5 N HC1, B = 58.93ml
Determination of Aldehyde value:
Of the many procedures which have been suggested for the determination of aldehyde and ketones, only four general methods have attained practical significance. These are bisulfite method, the neutral sulfite method, the phenylhydrazine method, and the hydroxylamine methods.
Hydroxylamine hydrochloride method:
Two important techniques have been developed, both based up on the use of hydroxylamine for the determination of aldehydes and ketones. The first makes use of a solution of hydroxylamine hydrochloride and subsequent neutralization with standardize alkali of the hydrochloric acid liberated by the reaction. The second technique makes use of a solution of hydroxylamine; after the reaction with the aldehydes or ketones the mixture is titrated with standardize acid. The later procedure is known as Stillman-Reed method. Both modifications are based upon the fundamental reaction:
Preparation of reagents:
a Preparation of bromophenol blue indicator:
44 mg of bromophenol blue indicator was dissolved in 96% ethanol.
b Preparation of 0.5 N Alcoholic KOH solutions:
Molecular weight of KOH=39.1+16+1 =56. 1g.
15g of KOH pellets was dissolved in 8ml of distilled water and the solution was mixed with 500 ml of 96% (v/v) ethanol. The solution was allowed to stand for several hours.The clear supernatant liquid was filtered off and the filtered solution was kept in amber coloured bottle at dark place for further use. Using phenolphthalein indicator, the strength of KOH was determined by titrating it (KOH) by 0.5 N HC1 Solution.
c Preparation of 0.5 N HC1 solution:
20.82ml cone. HC1 (12N) was taken from Burette in 500 ml volumetric flask and it was diluted up to the mark with distilled water.
d Recrystallization of NH2OH.HC1:
NH2OH.HCI crystals were dissolved in a minimum amount of rectified sprit by heating over steam bath and heating was continued for 2h and the solution allowed to cooling.The solid crystals of NH2OH.HCI formed were then filtered of by means of a suction pump. The residue was taken in a watch glass and then it was dried by inserting it in desiccators. Thus recryslallized NH2OH.HCl: was obtained.
e Preparation of 0.5 (N) Hydroxylamine hydrochloride (NH2OH.HC1) Solution:
About 9.0 g of recrystallized NH2OH.HCI was taken in a 250 ml volumetric flask and
was dissolved in 219 ml of 60% ethanol. About 2-3 ml of bromophenol blue indicator was added to the solution. Then 0.5 N KOI I alcoholic solution was added drop wise to make the solution greenish in colour, such that one drop of 0.5 (N) HC1 solution would change the colour into yellow. Then the resulting solution was made up to the mark of the volumetric flask with distilled water.
Standardization of alch. KOH by 0.5 (N) H.C1:
10 ml of HC1 was taken in a conical flask with the help of pipette. Few drops of 1% phenolphthalein added. The solution become colourless KOH solution was taken in a burette. KOH was added drop wise into the HC1 solution. At the end of the titration the colourless solution became pink.
The result is tabulated as follows:
Table: Titration data (Standardization of alch. KOH by 0.5 (N) HC1):
Observe, No. | HC1 solution (ml) | Burette reading , Volume of KOH solution (ml) | |||
| Initial reading | Final reading | Difference | Average volume | ||
1 | 10 | 0.0 | 11.2 | 11.2 | 11.1 |
2 | 10 | 0.0 | 11.1 | 11.1 | |
3 | 10 | 0.0 | 11.1 | 1.1.1 | |
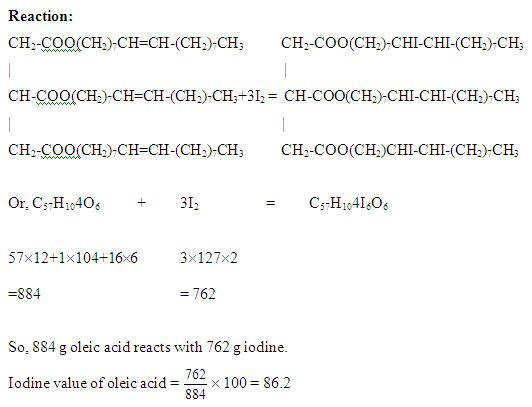
Determination of Iodine Value:
Iodine value is expressed in gm of iodine absorbed by 100 gms of oil or fat. It gives the indication of degree of unsaturation of the constituent oil and is thus a relative measure of the unsaturated bonds present in the oil. The iodine value is a characteristic of oil. Unsaturated compounds absorb iodine (in soluble form) and form saturated compounds. One double bond absorbs one molecule of iodine. The amount of iodine absorbed in percentage is the measure of unsaturation in the oil. Oils are classified as drying, semi- drying and non-drying on the basis of iodine value. The use of iodine numbers for the evaluation of essential oil has never attained practical significance. It has been shown frequently that the iodine numbers of many essential oil nary with the size of sample as well as with the period of contact with the reagent.
Unsaturated compounds absorb iodine as flows.
Of the many procedure that have been proposed for determination the Iodine value four methods are better known than all others. These are the methods of Wijs and Hanus, Hubl and Rosenmund, Kuhnuhenn method. The Wijs and Hanus method, especially the former, is the most widely used of all. The International union of pure and applied chemistry approved wijs, Hanus and Hubl methods for the iodine value determination. The iodine value of sample oil was determined hcre by using Hanus method.
Reagents required:
- 15 % KI Solution.
- N Na2S2O3 Solution
- 0.1N K2Cr2O7 Solution
- Starch indicator
- Hanus solution
- Pure iodine.
- Glacial acetic acid
- Solid sodium bicarbonate (NaHCO3)
- Bromine
- Chlorodorm (CHCl3)
- Solid KI
- Conc. HCl
Preparation of Reagents for iodine value:
a) 0.1 N Na2S2O3 solution preparation:
Sodium thiosulphate (Na2S2O3.5H2O) is readily obtainable in a state of high purity, but there is always some uncertainty as to the exact water content because of the efflorescent nature of the salt and for other reasons. The substance is therefore unsuitable as primary standard. It is a reducing agent by virtue of the half-cell reaction:
2S2O32- S4O62- + 2e-
The equivalent weight of sodium thiosulphate penta hydrate (Na2S2O3.5H2O) is 248 So, 24.8 g of A.R. crystallized sodium thiosulphate pentahydrate (Na2S2O3.5H2O) was taken in liter of volumetric flask and it was diluted up to the mark with distilled water. Two or three drops of chloroform added to it. The solution must be stored in amber or yellow glass Stoppard bottle. As Na2S2O3 is not a primary standard substance. It must be standardized by potassium dichromate (K2Cr2O7) solution that is a primary standard substance.
b) Preparation of 0.1N K2Cr2O7 solution:
Molecular weight of K2Cr2O7 = (39 ´2 + 52´2+16´7) g = 294g
Oxidation Number of K2Cr2O7 = 6
Equivalent weight of K2Cr2O7 = 294/6 = 49
1.2259g of K2Cr2O7 is required to prepare 0.lN K2Cr2O7 Solution in 250m1 water.
So, 1.2487 g of K2Cr2O7 was taken in 250 m1 volumetric flask and it was diluted up to
the mark with distilled water.
So, the strength of K2Cr2O7 Solution = ´ 0.10193 N
c) 15% KI solution preparation:
About 15 g of dry A.R. potassium iodide was weighed out and then it was dissolved in l00ml of distilled water in the titration vessel. It was stored in amber colour glass Stoppard bottle.
d) Preparation of starch solution:
1 gm of soluble starch was poured in 100 m1 of distilled water and stirred rapidly. It was heated till boiling and kept on boiling I minute. The solution was allowed to cool and 2-3 g of potassium iodide (K1) was added. For long storage 1.0 g of boric acid was added and taken a clear solution from it. The solution was kept in a Stoppard bottle and preserved in a refrigerator at 4° to 10° C.
e) Preparation of Hanus solution:
13 g of pure resublimed iodine was added to glacial acetic acid by warming over water bath. When the iodine was completely dissolved the solution was cooled and diluted to 1000 ml with glacial acetic acid. Now 3 ml of pure (sulfur free) Br2 was added to the solution stored it in amber colour Stoppard bottle. The whole operation was conducted in a fume hood.
Precaution:
Acetic acid has a relatively high temperature coefficient of expansion so that blank titration made at different periods of the day may vary significantly due to changes in temperature within the laboratory.
Standardization of Na2S2O3 solution by 0.1 N K2Cr2O7 solutions:
l0 ml l5% potassium iodide (KI) solution, 2 g of sodium bicarbonate (NaHCO3) were taken in a 500 m1 Stoppard conical flask and l00 ml of distilled water was added in it to dissolve the solids. Then 6ml of concentrated HCI added. 10 ml of 0.1 N K2Cr2O7added to the conical flask. The conical flask was kept in a dark room for fifteen minutes. The solution, became deep brown in colour. Then 200 ml of the distilled water was added to the solution. Earlier sodium thiosulfate (Na2S2O3)solution was taken in a burette.Titration was carried out by adding sodium thiosulfate (Na2S2O3) solution from burette to the solution kept in conical flasks. When deep brown colour changed into yellowish green colour, then 1 ml starch solution was added. The colour quickly changed into deep blue, at this stage titration was carried out with special care by adding sodium Thiosulfate (Na2S2O3) solution drop by drop. At tl-re end point the colour of the solution changed greenish blue to light green.
Table: Titration data (Standardization of Na2S2O3 solution):
Observe No.
| Volume of K2Cr2O7 solution, ml | Burette reading ,volume of Na2S2O3 solution (ml) | |||
| Initial reading | Final reading | Difference | Average
| ||
1 | 10 | 0.0 | 7.1 | 7.1 | 7.0 |
2 | 10 | 6.9 | 6.9 | 6.9 | |
3 | 10 | 7.0 | 7.0 | 7.0 | |
Calculation:
As we know, V1S1 = V2 S2
Here, Vl = Volume of K2Cr2O7 solution = 10 ml
V2 = Volume of Na2S2O3 solution = 7.0 ml
S1 = Strength of K2Cr2O7 solution = 0.10193 N
Procedure for the determination of iodine value:
About 0.5 gm of fatty oil was taken in a well Stoppard conical flask. The oil was dissolved in 10 ml of chloroform. 25 ml of Hanus solution was added to the Stoppard conical flask from a pipette and the solution was allowed to stand for half an hour in dark place with occasional shaking. At the end of this period, 100 ml of distilled water was added followed by 10 ml of l5% KI solution.
The solution was titrated with standard 0.1 N Na2S2O3 solutions. The sodium thiosulphate was gradually added with constant shaking, until yellow colour of the solution was almost disappeared. Few drops of starch solution were added and the titration was continued until the dark violate colour was entirely disappeared.
The blank determination was also carried out observing the same condition but omitting the oil.
Calculation method:
Iodine value was determined by following formula:
Iodine value =
Where, B = Volume of Na2S2O3 required for the blank titration
S = Volume of Na2S2O3, required for the sample
N = Strength of the Na2S2O3 solution
W = Weitght of the fatty oil taken in g
Determination of Unsaponifiable Matter of fatty oil:
Oil derived from natural sources contain small amount of dissolved Unsaponifiable matter. Mineral oil contamination, higher aliphatic alcohols, sterols, pigments and hydrocarbons are the materials of this nature. The determination of unsaponifiable matter is important to know the purity and quality of the sample. The term “unsaponifiable matter” is now generally understood to indicate that material present in oils and fats which after saponification of the oil or lat by caustic alkali and extraction by the solvent specified (under the conditions detailed in the description of the method of the society of public analysis and other analytical chemists) remains non volatile on drying at 80°C unsaponifiable matter as defined above includes, interalia, hydrocarbons and higher alcohol, cholesterol and phytosterol. The method of determination aims at the exclusion of free fatty acids, soap free fat, mineral matter and glycerol and readily volatile substances. The methods are generally based on the preliminary saponification of the oil with caustic alkali and subsequent extraction of the soap so formed by means of solvent. Later on aqueous alcoholic solution of the soap was extracted with light ptroleum; the extract was washed with water and alkali to remove soap and evaporated to yield the unsaponifiable matter.
- · Reagents required:
- 0.5N Alcoholic KOH solution, colour not darker than pale yellow.
- Diethyl ether
- 1% Phenolphthalein
Procedure for the determination of Unsaponifiable Matter:
An accurately weighed 1.0 g of oil or fat was taken into a 250 ml R.B. flask and 25 ml of alcoholic KOH was added to it. The flask was attached to a refluxing condenser and heated on a boiling water bath for an hour. The contents of flask were occasionally stirred to mix the solution properly and to ensure complete saponification.
After completion of saponification, the flask was removed from the bath, the condenser was detached and the content of the flask was transferred to a 250 ml separating funnel. The solution was washed with 50 ml of distilled water. Then the
flask was rinsed with 50 ml of diethyl ether and this ether was poured cautiously into the separating funnel. The funnel was covered and shaken vigorously while the contents were still slightly warm. After shaking for about 30 seconds the funnel was then suspended and left stationary till there appeared two distinct layers in the liquid mixture. The upper ethereal layer was collected in a R.B. flask by transferring the lower aqueous layer of the solution into another flask. The aqueous layer of the soap solution was extracted twice more with ether in the similar way. The ether extract were then combined 30 ml of distilled water and then 20 ml of 0.5 N aqueous KOH solutions.
After one or other of these preliminary treatments, the ethereal solution was washed twice with 20 ml of water. It was shacked vigorously on each occasion. And then successively washed with 20 ml of 0.5 N aqueous KOH solutions, 20 ml of distilled water and again with 20 ml of 0.5 N aqueous KOH solution and at least twice more with 20 ml of distilled water. Washing with water was continued until the wash water no longer turned pink, on addition of phenolphthalein indicator. The ethereal solution was transferred to a sample flask and ether was evaporated from the solution. Then the flask being almost entirely immersed, hold obliquely and rotated in a boiling water bath. When the flasks become dried and weight (with its contents) was constant at 80°C.The extract was dissolved in 10 ml of freshly boiled and neutralized 95% ethanol and titrated with the 0.1 N alcoholic sodium hydroxide solutions, using phenolphthalein idicator.
Calculation method:
The Unsaponified matter was found by the following formula.
Unsaponified matter (% by weight) =
Where,
Wl= Weight of residue (g).
W = Weight of sample taken (g)
Table-: Calculation table for unsaponifiable matter of fatty oil:
a) China Ginger:
Observe No.
| Weight of Sample, W g
| Weight of Residue, W1 g | Unsaponifiable matter %, | Average Unsaponified matter %
|
1 | 1.1 | 0.38 | 34.5 |
34.03 |
2 | 1.05 | 0.36 | 34.3 | |
3 | 1.2 | 0.40 | 33.3 |
Result: The average unsaponifiable matter of China Ginger: 34.0
b) Chittagong Ginger:
Observe No.
| Weight of Sample,W g
| Weight of Residue, W1 g
| Unsaponifiable matter %, | Average Unsaponified matter %
|
1 | 1.0 |
0.36 |
36 | 36.06 |
2 | 1.0 | 0.36 |
36 | |
3 | 1.05 | 0.38 |
36.2 |
Result: The Unsaponifiable matter Chittagong Ginger: 36.06
Determination of peroxide Value of fatty oil:
The peroxide value is the number of mili-equivalent of active oxygen that expresses the amount of peroxide contained in 1000 g of the substance or peroxide oxygen per 1 kilo-gram of fat or oil. The sample is treated with potassium iodide and the iodine which was liberated by the peroxides was treated with sodium thiosulphate solution.
The Peroxide value of an oil or fat is used as a measurement of the extent to which rancidity reactions have occurred during storage. Other methods are available but peroxide value is the most widely used.
The double bonds found in fats and oils play a role in autoxidation. Oils with a high degree of unsaturation are most susceptible to autoxidation. The best test for autoxidation (oxidative rancidity) is determination of the peroxide value. Peroxides are intermediates in the autoxidation reaction. Autoxidation is a free radical reaction involving oxygen that leads to deterioration of fats and oils which form off-flavours and off-odours. Peroxide value, concentration of peroxide in an oil or fat, is useful for assessing the extent to which spoilage has advanced.
The peroxide value is determined by measuring the amount of iodine which is formed by the reaction of peroxides (formed in fat or oil) with iodide ion.
2I– + H2O + ROOH = ROH + 2OH– + I2
Note that the base produced in this reaction is taken up by the excess of acetic acid present. The iodine liberated is titrated with sodium thiosulphate.
2S2O32- + I2 = S4 +O62- + 2I–
The indicator used in this reaction is a starch solution where amylose forms a blue to black solution with iodine and is colourless where iodine is titrated.
Correlation of rancid taste and peroxide value depends on the type of oil and is best tested with taste panels. The odours and flavors associated with typical oxidative rancidity are mostly due to carbonyl type compounds. The shorter-chain aldehydes and ketones isolated from rancid fats are due to oxidative fission and are associated with advanceed stages of oxidation. The carbonyl-type compounds develop in low concentrations early in the oxdative process.
Traditionally this was expressed in units of milliequivalents, although if we are using SI units then the appropriate option would be in millimoles per kilogram (N.B. 1
millimole = 2 milliequivalents). Note also that the unit of milliequivalent has been commonly abbreviated as mequiv or even as meq.
Reagents used:
- 0.lN K2Cr2O7 solution
- 15 % KI solution
- Chloroform-Acetic acid solution with the ratio of 2: 3.
- Saturated KI solution.
- Conc. HCI
- Starch solution
- Na2S2O3 solution (a few strength)
- Solid sodium bicarbonate (Na2HCO3)
Preparation of reagents:
a) 0.05 N Na2S2O3 solution preparation:
Sodium thiosulphate (Na2S2O3.5H2O) is readily obtainable in a state of high purity, but there is always some uncertainty as to the exact water content because of the efflorescent nature of the salt and for other reasons. The substance is therefore unsuitable as primary standard. It is a reducing agent by virtue of the half-cell reaction:
2S2O32- S4O62- + 2e-
The equivalent weight of sodium thiosulphate penta hydrate (Na2S2O3.5H2O) is 248. So, 24.8 g of A.R. crystallized sodium thiosulphate pentahydrate (Na2S2O3.5H2O) was
taken in liter of volumetric flask and it was diluted up to the mark with distilled water. Two or three drops of chloroform added to it. The solution must be stored in amber or yellow glass Stoppard bottle.As Na2S2O3 is not a primary standard substance. It must be standardized by potassium dichromate (K2Cr2O7) solution that is a primary standard substance.
b) Preparation of 0.lN K2Cr2O7 solution:
Molecular weight of K2Cr2O7 = (39 ´2 + 52´2+16´7) gm = 294g
Oxidation Number of K2Cr2O7 = 6
Equivalent weight of K2Cr2O7 = 294/6 = 49
1.2259g of K2Cr2O7 is required to prepare 0.lN K2Cr2O7 Solution in 250m1 water.
So, 1.2487 gm of K2Cr2O7 was taken in 250m1 volumetric flask and it was diluted up to the mark with distilled water.
So the strength of K2Cr2O7 Solution = N
c) 15% KI solution preparation:
About 15 g of dry A.R KI was weighed out and then it was dissolved in 100ml distilled water in the titration vessel. It was stored in amber colour glass stoppard bottle.
d) Preparation of starch solution:
1 g of soluble starch was poured in 100 m1 of distilled water and stirred rapidly. It was heated till boiling and kept on boiling 1 minute. The solution was allowed to cool and 2-
3 g of potassium iodide (K1) was added. For long storage 1g of boric acid was added and taken a clear solution from it. The solution was kept in a Stoppard bottle and
preserved in a refrigerator at 4° to 10° C.
e) Preparation of chloroform-Acetic acid 2:3 solution:
30 ml acetic acid and 20 ml chloroform was added in a 100 ml cylinder.
f) Preparation of saturated KI solution preparation:
100 ml distilled water was taken in a clean beaker. Gradually KI was added. At this
time heat was applied for dissolving. As long as KI was dissolved in water further KI was added. Soon it was seen that no potassium iodide was dissolving in water any way.This was saturation point of KI then KI addition was stopped.
Procedure for the determination of peroxide value:
0.5 g of sample was placed in a 250 ml glass Stoppard conical flask. 7.5 ml of Chloroform-Acetic acid 2:3 solution added and shaken until the sample was dissolved.Then 0.25 ml of saturated Potassium iodide (KI) solution and few drops of conc. HCl also added to it. Then the solution had been shaken for at least 1 minute. 15 ml distilled water was added to the solution and it was titrated carefully with 0.055 N Sodium thiosulphate solutions until the brown colour was faded to pale yellow. 0.5 ml of starch solution was then added and the titration was completed with continuous shaking when the blue colour just disappeared at the end point.
Calculation method:
The peroxide value of the fatty oil was calculated by the following formula:
Peroxide value = ,The strength of Na2S2O3 solution,S = 0.081 N
Table-: Titration data for peroxide value of fatty oil.
a) China Ginger:
Observe No.
| Weight of sample, W g | Burette reading, Volume of Na2S2O3 Solution, V Na2S2O3 ml | Peroxide value, | Average Peroxide value
| ||
IBR | FBR | Difference | ||||
1 | 0.56 | 0.0 | 0.2 | 0.2 | 28.9 | 31.46 |
2 | 0.75 | 0.0 | 0.3 | 0.3 | 32.4 | |
3 | 0.49 | 0.0 | 0.2 | 0.2 | 33.1 | |
Result: The average peroxide value of China Ginger: 31.46
a) Chittagong Ginger:
Observe No.
| Weight of sample, W g | Burette reading, Volume of Na2S2O3Solution, V Na2S2O3 ml | Peroxide value, | Average Peroxide value
| ||
IBR | FBR | Difference | ||||
1 | 0.6 | 0.0 | 0.25 | 0.25 | 33.7 |
32.62 |
2 | 0.51 | 0.0 | 0.2 | 0.2 | 31.76 | |
3 | 0.50 | 0.0 | 0.2 | 0.2 | 32.4 | |
Result: The average peroxide value of Chittagong Ginger: 32.62
Discussion on mineral elemental Analysis of Zingiber officinale Roscoe (Ginger):
The mineral content was determined by AAS (Atomic absorption Spectrometer). Model no: SpectrAA 55B, made by Astralia. Instrument type: UV-Vib Spectro photomoter mineral element 50 concentration was detected on dry matter basis.
Mineral contents of Zingiber officinale (Ginger):
Mineral content such as Na, Mg, P, S, K, Ca, Cr, Mn, Fe, Ni, B, Pb, Cd etc. were determined in its fresh ginger powder. Plants and its rhizome were collected from Bangladesh and China. The result of element analysis of its rhizome was determined by AAS (Atomic absorption Spectrometry) method in g/l00g dry weight basis of the sample. The element analysis of Zingiber officinale Roscoe (ginger) rhizome can be compared with different countries. The result of minerals content of ginger rhizome of Bangladesh and china appeared as element basis in Table-48
The total concentration of macro and micro elements were measured in ginger rhizome from two different cuntries of Bangladesh and China by AAS (Atomic Absorption spectrometry) for the first time in Bangladesh by the authors. Precision of the measurements was taken by analysis of two samples of Bangladesh and China.
Table-: Elemental analysis in percent comparatively (on dry matter basis) of Zingiber officinale Roscoe (Ginger) rhizome of Bgladesh and China
No. | Component as Element | Name of the region | |
China Ginger | Chittagong Ginger | ||
1 | Na | 0.34 % | 0.24 % |
2 | Mg | 0.809 % | 0.611 % |
3 | P | 1.12 % | 1.20 % |
4 | S | 0.130 % | 0.050 % |
5 | K | 0.68 % | 0.244 % |
6 | Ca | 2.43 % | 1.70 % |
7 | Mn | 15.51µg/L | 17.26µg/L |
8 | Fe | 2.48µg/L | 3.69µg/L |
9 | Ni | 3.0µg/L | 1.70µg/L |
10 | B | 40.0µg/L | 35µg/L |
11 | Cd | 1.20µg/L | 1.10µg/L |
12 | Pb | 1.90µg/L | 3.50µg/L |
13 | Cr | Trace | Trace |
Biological Studies
Antimicrobial Activity
The medicinal value of plants lies in some chemical substrates that produce a definitive physiological action on the human body. The most important antimicrobial compounds of plants are alkaloids, flavonoids, tannins and phenolic compounds. The increasing prevalence of multi-drug resistant strains of bacteria and the recent appearance of strains with reduced susceptibility to antibiotics raised the specter of ‘untreatable’ bacterial infections and adds urgency to the search for new infection-fighting strategies.
Contrary to synthetic drugs, antimicrobials of plant origin usually are not associated with many side effects and have an enormous anti-infective potential in numerous infectious diseases. There is an ever-increasing demand for plant-based therapeutics in both developing and developed countries due to a growing recognition that they are natural products, non-narcotic and in most cases, easily available at affordable prices; they also have no side effects.
In general, bacteria and fungi have the genetic ability to transmit and acquire resistance to drugs, which are utilized as therapeutic agents. The use of plant extracts and phytochemicals, with established antimicrobial properties, could be of great significance in preventive and/or therapeutic approaches. The increasing clinical implications of drug resistant fungal and bacterial pathogens have lent additional urgency to antimicrobial drug research. The antimicrobial screening which is the first stage of antimicrobial drug research is performed to ascertain the susceptibility of various fungi and bacteria to any agent. This test measures the ability of each test sample to inhibit the in vitro fungal and bacterial growth. This ability may be estimated by either of the following three methods.
i) Disc diffusion method
ii) Serial dilution method
iii) Bioautographic method
But there is no standardized method for expressing the results of antimicrobial screening. Some investigators use the diameter of zone, of inhibition and or the minimum weight of extract to inhibit the growth of microorganisms. However, a great number of factors viz., the extraction methods, inoculum volume, culture medium composition, pH and incubation temperature can influence the results.
Among the above mentioned techniques the disc diffusion is a widely accepted in vitro investigation for preliminary screening of test agents which may possess antimicrobial activity. It is essentially a quantitative or qualitative test indicating the sensitivity or resistance of the microorganisms to the test materials. However, no distinction between bacteriostatic and bacteriocidal activity can be made by this method.
Principle of Disc Diffusion Method
Solutions of known concentration (mg/ml) of the test samples are made by dissolving measured amount of the samples in calculated volume of solvents. Dried and sterilized filter paper discs (6 mm diameter) are then impregnated with known amounts of the test substances using micropipette. Discs containing the test material are placed on nutrient agar medium uniformly seeded with the test microorganisms. Standard antibiotic discs and blank discs (impregnated with solvents) are used as positive and negative control. These plates are then kept at low temperature (4 0C) for 24 hours to allow maximum diffusion. During this time dried discs absorb water from the surrounding media and then the test materials are dissolved and diffused out of the sample disc. The diffusion occurs according to the physical law that controls the diffusion of molecules through agar gel. As a result there is a gradual change of test materials concentration in the media surrounding the discs.
The plates are then incubated at 37°C for 24 hours to allow maximum growth of the organisms. If the test materials have any antimicrobial activity, it will inhibit the growth of the microorganisms and a clear, distinct zone of inhibition will be visualized surrounding the medium. The antimicrobial activity of the test agent is determined by measuring the diameter of zone of inhibition expressed in millimeter.
The experiment is carried out more than once and the mean of the readings is calculated.
In the present study essential oil were tested for antimicrobial activity by disc diffusion method. Other pure compounds could not be tested due to scarcity of samples.
Experimental
Apparatus and Reagents
| Filter paper discs | Petri dishes | Inoculating loop |
| Sterile cotton | Sterile forceps | Spirit burner |
| Micropipette | Screw cap test tubes | Nose mask and hand gloves |
| Laminar air flow hood | Autoclave | Incubator |
| Refrigerator | Nutrient Agar Medium | Ethanol |
| Chloroform |
Test Organisms
The bacterial and fungal strains used for the experiment were collected as pure cultures from the Institute of Nutrition and Food Science (INFS), University of Dhaka. Both Gram positive and Gram-negative organisms were taken for the test and they are listed in the Table-49
Table: List of test bacteria and fungi.
Gram positive bacteria | Gram negative bacteria | Fungi |
| Bacillus sereus
Bacillus megaterium
Bacillus subtilis
Staphylococcus aureus
Sarcina lutea | Escherichia coli Pseudomonas aureus Salmonella paratyphi Salmonella typhi Shigella dysenteriae Shigella boydii Vibrio mimicus Vibrio parahemolyticus | Candida albicans Aspergillus niger Sacharomyces cerevaceae |
Culture medium and its composition
Nutrient agar medium (DIFCO) is used normally to demonstrate the antimicrobial activity and to make subculture of the test organisms.
Nutrient agar medium
Sodium chloride 0.5 gm
Bacto yeast extract 1.0 gm
Bacto agar 2.0 gm
Preparation of medium
To prepare required volume of this medium, calculated amount of each of the constituents was taken in a conical flask and distilled water was added to it to make the required volume. The contents were heated in a water bath to make a clear solution. The pH (at 25 °C) was adjusted at 7.2 – 7.6 using NaOH or HC1 10 ml and 5 ml of the medium was then transferred in screw cap test tubes to prepare plates and slants respectively. The test tubes were then capped and sterilized by autoclaving at a temperature of 121°C and a pressure of 15-lbs./sq. inch for 20 minutes. The slants were used for making fresh culture of bacteria and fungi that were in turn used for sensitivity study.
Sterilization procedures
In order to avoid any type of contamination and cross contamination by the test organisms the antimicrobial screening was done in Laminar Hood and all types of precautions were highly maintained. UV light was switched on one hour before working in the Laminar Hood. Petridishes and other glasswares were sterilized by autoclaving at a temperature of 121 °C and a pressure of 15-lbs./sq. inch for 20 minutes. Micropipette tips, cotton, forceps, blank discs etc. were also sterilized.
Preparation of subculture
In an aseptic condition under laminar air cabinet, the test organisms were transferred from the pure cultures to the agar slants with the help of a transfer loop to have fresh pure cultures. The inoculated strains were then incubated for 24 hours at 37 °C for their optimum growth. These fresh cultures were used for the sensitivity test.
Preparation of the test plates
The test organisms were transferred from the subculture to the test tubes containing about 10 ml of melted and sterilized agar medium with the help of a sterilized transfer loop in an aseptic area. The test tubes were shaken by rotation to get a uniform suspension of the organisms. The bacterial and fungal suspension was immediately transferred to the sterilized petridishes. The petridishes were rotated several times clockwise and anticlockwise to assure homogenous distribution of the test organisms in the media.
Preparation of discs
Three types of discs were used for antimicrobial screening.
a) Standard discs
These were used as positive control to ensure the activity of standard antibiotic against the test organisms as well as for comparison of the response produced by the known antimicrobial agent with that of the test sample. In this investigation, Kanamycin (30mg/disc) standard disc was used as the reference.
b) Blank discs
These were used as negative controls which ensure that the residual solvent (left over the discs even after air-drying) and the filter paper were not active themselves.
c) Preparation of sample discs with test samples
Measured amount of each test sample was dissolved in specific volume of solvent to obtain the desired concentrations in an aseptic condition. Sterilized metrical BBL, Cocksville, USA) filter paper discs were taken in a blank petridish under the laminar hood. Then discs were soaked with solutions of test samples and dried.
d) Preparation of sample discs with test samples of Zingiber Officinale Roscoe.
Essential oils were tested for antimicrobial activity against a number of both gram positive, gram negative bacteria and fungi.
1) Test samples dosages:
The amount of sample per disc were 100, 200 and 400 mg.
Preparation and application of the test samples
The test samples were weighed accurately and calculated amounts of the solvents were added accordingly using micropipette to the dried samples to get desired concentrations. The test samples were applied to previously sterilized discs using adjustable micropipette under aseptic conditions.
Diffusion and Incubation
The sample discs, the standard antibiotic discs and the control discs were placed gently on the previously marked zones in the agar plates pre-inoculated with test bacteria and fungi. The plates were then kept in a refrigerator at 4 °C for about 24 hours upside down to allow sufficient diffusion of the materials from the discs to the surrounding agar medium. The plates were then inverted and kept in an incubator at 37°C for 24 hours.
Determination of antimicrobial activity by the zone of inhibition
The antimicrobial potency of the test agents are measured by their activity to prevent the growth of the microorganisms surrounding the discs which gives clear zone of inhibition. After incubation, the antimicrobial activities of the test materials were determined by measuring the diameter of the zones of inhibition in millimeter with a transparent scale.
| Table-50: Antimicrobial activity of the Zingiber Officinale Roscoe (Ginger) essential oil (100μg/disc) and ciprofloxacin (5 μg/disc).
| ||||
Test microorganisms | China variety (mm) | Chittagong Variety (mm) | C.F (mm) | |
| Gram positive bacteria | Bacillus megaterium | 15 | 17 | 45 |
| Bacillus subtilis | 16 | 17 | 46 | |
| Staphylococcus aureus | 18 | 18 | 46 | |
| Sarcina lutea | 18 | 18 | 45 | |
| Bacillus sereus | 16 | 15 | 45 | |
| Gram negative bacteria | Escherichia coli | 18 | 18 | 46 |
| Pseudomonas aureus | 18 | 18 | 46 | |
| Salmonella paratyphi | 18 | 18 | 46 | |
| Salmonella typhi | 18 | 18 | 45 | |
| Shigella dysenteriae | 18 | 18 | 46 | |
| Shigella boydii | 16 | 16 | 45 | |
| Vibrio mimicus | 18 | 18 | 46 | |
| Vibrio parahemolyticus | 18 | 18 | 46 | |
Fungi | Candida albicans | 18 | 18 | 45 |
| Aspergillus niger | 18 | 18 | 46 | |
| Sacharomyces cerevaceae | 18 | 18 | 45 | |
| C.F.: ciprofloxacine. | ||||
Antioxidant activity
Evaluation of antioxidant activity
Majority of diseases/disorders are mainly linked to oxidative stress due to free radicals. Antioxidant can interfere with the oxidation process by reacting with free radicals chelating, catalytic metals and also by acting as oxygen scavengers. There is an increasing interest in the antioxidant effects of compounds derived from plants which could be relevant in relation to their nutritional incidence and their role in health and diseases. A number of reports on the isolation and testing of plant derived antioxidants have been described during the past decade. The medicinal properties of plants have been investigated in the recent scientific developments throughout the world, due to their potent antioxidant activities, no side effects and economic validity.
Antioxidant compounds in foods play important roles as health-protecting factors. The number of contribution to isolation methods, techniques and activity testing of plant-origin antioxidants has significantly increased in recent years. Oxidation is one of the major causes of chemical spoilage, resulting in rancidity and/or deterioration of the neutritional quality, color, flavour, texture and safety of foods. There is at present increasing interest in the world, both in the industry and in scientific research of medicinal plants because of their strong antioxidant activities, no side effects and economic viability. Which exceed many currently used natural and synthetic antioxidant. These properties are due to many substances, including some vitamins, flavonoids, terpenoids, carotenoids, phytoesterogens, minerals etc.
Different synthetic antioxidant such as tert-butyl-1-hydroxytolune (BHT), butylated hydroxyanisole(BHA), Propyl gallate(PG) and tert-butylhydroquinone(TBHQ) used as food additives to increase self life are known to have not only toxic and carcinogenic effects but also abnormal effects on enzyme systems 127. Therefore, the interest in natural antioxidant, especially of plant origin, has greatly increased in recent years. The determination of scavenging stable DPPH was a very fast method to evaluate the antioxidant activity of the substance. The purpose of this study was to evaluate different extractives of Zingiber Officinale Roscoe bark as new potential sources of natural antioxidant by DPPH method.
Antioxidant activity: DPPH assay Principle
An antioxidant is a molecule or even a single atom with an extra electron. These extra electrons stabilize free radicals. The free radical scavenging activities (antioxidant capacity) of the plant extracts on the stable radical 1, 1-diphenyI-2-picryIhydrazyl (DPPH) were estimated by the method of Brand-Williams 128. The determination of scavenging stable DPPH was a very fast method to evaluate the antioxidant activity of the extracts. With this method it was possible to determine the antiradical power of an antioxidant activity by measurement of the decrease in the absorbance of DPPH at 517 nm. Resulting from a color change from purple to yellow the absorbance decreased when the DPPH was scavenged by an antioxidant, through donation of hydrogen to form a stable DPPH molecule. In the radical form this molecule had an absorbance at 517 nm which disappeared after acceptance of an electron or hydrogen radical from an antioxidant compound to become a stable diamagnetic molecule.
The odd electron in the DPPH free radical gives a strong absorption maximum at 517 nm and is purple in color. The color turns from purple to yellow as the molar absorptivity of the DPPH radical at 517 nm reduces from 9660 to 1640 when the odd electron of DPPH radical becomes paired with hydrogen from a free radical scavenging antioxidant to form the reduced DPPH-H. The resulting decolorization is stoichiometric with respect to number of electrons captured.
Materials & Methods
DPPH was used to evaluate the free radical scavenging activity (antioxidant potential) of various compounds and medicinal plants.
Materials:
| 1,1-diphenyl-2-picrylhydrazyl (DPPH) | Analytic Jene Spekel 1300 UV-spectrophotometer |
| Tert-butyl-1-hydroxytolune (BHT) | Amber reagent bottle |
| Beaker (100 & 200 mL ) | Test tube0 |
| Ascorbic acid | Methanol |
| Distilled water | Light-proof box |
| Micropipette (50-200 µL) | Amber reagent bottle |
| Pipette (5 mL & 10 mL ) |
Control preparation for antioxidant activity measurement
Ascorbic acid (ASA) and tert-butyl-1-hydroxytoluene (BHT) was used as positive control. Calculated amount of ASA and BHT were dissolved in methanol to get a mother solution having a concentration 1000 µg/ml. Serial dilution was made using the mother solution to get different concentration ranging from 500.0 to 0.977mg /ml.
Test sample preparation
Calculated amount of different extractives were measured and dissolved in methanol to get the mother solution (Conc. 1000 µg/ml). Serial dilution of the mother solution gave different concentration ranging from 1000.0 to 1.953125 mg /ml which were kept in the marked flasks.
DPPH solution preparation
20 mg DPPH powder was weighed and dissolved in methanol to get a DPPH solution having a concentration 20 µg/ml. The solution was prepared in the amber reagent bottle and kept in the light proof box.
Assay of free radical scavenging activity
2.0 ml of a methanol solution of the sample (extractives/ control) at different concentration (1000 mg /ml to 1.9531 mg /ml) were mixed with 3.0 ml of a DPPH methanol solution (20 mg /ml). After 30 min reaction period at room temperature in dark place the absorbance was measured at 517 nm against methanol as blank by UV spectrophotometer. Inhibition of free radical DPPH in percent (1%) was calculated as follows:
% inhibition =(1-Asample/ Ablank)×100
Where Ablank is the absorbance of the control reaction (containing all reagents except the test material).The experiments were performed thrice and the results were expressed as mean ±SD in every cases.
Extract concentration providing 50% inhibition (IC50) was calculated from the graph plotted inhibition percentage against extract concentration.
Results and discussion
On the basis of information found in the literature, we have planned and formulated the schemes of the research works (Scheme-1.1) on Zingiber officinale Roscoe (Ginger) rhizome. The experimental results, data, found on the basis of the conducted experiments are tabulated and discussed as follows in the comparison with the previous reported values of Bangladesh and China.
Proximate Analysis of Zingiber officinale Roscoe (Ginger) rhizome:
The proximate analysis like moisture, dry matter, ash, crude fiber, protein, carbohydrates, food energy, index of Zingiber officinale Roscoe (Ginger) rhizome of Bangladesh and China were determined by the conventional methods described in Section-3.1 to 3.8.
Discussion on proximate analysis of Zingiber officinale Roscoe (Ginger) rhizome of Bangladesh and China.
The proximate analysis of Zingiber officinale Roscoe (Ginger) rhizome can be compared with different countries. The comparative result of the proximate analysis of rhizome of Bangladesh and China appears in Table -66
In our study some variation is observed in our data. These variation may be due to on such factors as type of genetic variety, maturity, collection time, climatic condition in geographical location, composition of the soil, water, fertilizer used as well as permissibility, selectivity and absorbility of plants for the uptake of these parameter. All the effects caused the final level of proximate analysis in a plant. So far we aware till now the proximate analysis of Zingiber officinale Roscoe (Ginger) rhizome have not been investigated in Bangladesh by using modern analytical techniques and anywhere and were not reported earlier
Table-: Comparative study on the Proximate Analysis of Zingiber officinale Roscoe (Ginger) of Bangladesh and China.
Parameter of proximate analysis (g/l00g) | Sample collected from | |
China Ginger | Chittagong Ginger | |
Month of collection | November,2010 | December,2010 |
Maturity | Mature | mature |
Moisture | 81.90 | 79.41 |
Dry Matter | 18.09 | 20.59 |
Total ash | 7.1 | 8.13 |
Acid soluble ash | 82.4 | 79.6 |
Acid insoluble ash | 17.5 | 20.4 |
Water soluble ash | 52.6 | 55.7 |
Water insoluble ash | 47.3 | 44.2 |
Organic matter | 92.9 | 91.86 |
Crude fiber | 1.45 | 1.13 |
Nitrogen | 0.90 | 0.97 |
Protein | 5.6 | 6.1 |
Carbohydrates | 3.07 | 3.25 |
Food energy,(cal/gm) | 42.48 | 55.22 |
IR Analisis of Zingiber officinale Roscoe (Ginger):
Infrared (IR) spectroscopy is due to vibrational motions of the molecules. Vihralional energies are quantized.Thus when a molecule undergoes transition from a lower vibrational level to a higher vibrational level, it absorbs.IR radiation. It is well known that substances in all physical states such as solid. Liquid and gases have vibrational motions. Thus one should expect that all the substances should absorb IR radiation.
Section-3.8
The comparative result of the IR analisis of rhizome of fresh ginger powder of Bangladesh of China appears in Table-67 &.68
By IR analisis I found the flowing funtional groups on the sursace of China ginger & Chittagong ginger. Which are given in the flowing Table-67 &.68
Table-: China Ginger
Frequency cm-1
| 2852 cm-1 | – C-H (in alkenes) |
| 2341 cm-1 | – C C-(in alkynes) |
| 1635 cm-1 | – C = C- (in alkynes) |
| 1515 cm-1 | – C = C – (in aromatic rings) |
| 1029 cm-1 | > C = C< (in alkenes) |
Table-: Chittagong Ginger
Frequency cm-1
| 3263 cm-1 | Hydrogen banded alcohols, Phenols |
| 2854 cm-1 | – C-H (in alkenes) |
| 2345 cm-1 | – C C- (in alkynes) |
| 1636 cm-1 | – C = C – (in alkynes) |
| 1515 cm-1 | – C = C – (in aromatic rings) |
| 1153 cm-1 | – C -N- (in amine) |
| 1029 cm-1 | – C – O – (in alcohol, ether etc) |
| 939 cm-1 | > C = C < (in alkenes) |
Discussion on the essential oil of Zingiber officinale Roscoe (Ginger):
The essential oil of Zingiber officinale Roscoe (Ginger) was extracted by steam distillation according to the procedures described in Section-4.5 And Scheme-4.1. It was found that the Ginger contained 0.203% to 0.204% of essential oil was determined (Table-14) on the fresh weight basis (g/l000g), whereas Guenther found no reports of essential oil from the Zingiber officinale Roscoe (Ginger) but in Wealth of china found up to 0.203% of essential oil .
Discussion on the physical properties of the essential oil of Zingiber officinale Roscoe (Ginger):
The physical characteristics such as colour, appearance, specific gravity, optical rotation, solubility, refractive index of the essential oil were determined by conventional methods described in Section-4.6. The result of the physical properties of Zingiber officinale Roscoe (ginger) rhizome essential oil of Bangladesh and China appeared in Table-69
The slight variation of this oil content and the composition of the essential oil depend on several factors such as genotype, stage of maturity, cultivation peculiarities, soil composition and climatic differences in various geographical locations. Fluctuation of the oil composition can impart change in the organoleptic properties of the plant belonging to the botanical species and variety. So far we aware till now no systematic investigation on the Chemical composition of the essential oil of Zingiber officinale Roscoe (Ginger) rhizome have not been investigated in Bangladesh by using modern analytical techniques.
Comparative study of the chemical characteristics Zingiber officinale Roscoe (Ginger) rhizome essential oil of Bangladesh and China :
Chemical characteristics of the oil such as acid value. ester value, were determined by the conventional methods described in Section – 4.7 The result of the chemical characteristics of Zingiber officinale Roscoe (Ginger) rhizome essential oil of Bangladesh and China appeared in Table -70
Table-: Chemical characteristics of essential oil Zingiber officinale Roscoe (Ginger):
Chemical Characteristics | Sample collected from | ||
China Ginger | Chittagong Ginger | ||
Acid value | 6.5 | 8.7 | |
Ester value | 31.06 | 33.56 | |
Discussion on GC-MS analysis of the essential oil:
In the present study, essential oil Zingiber officinale Roscoe (Ginger) rhizome has been extracted from and isolated 11 compounds from the essential oil of two different regions in Bangladesh and China they were identified and quantified by GC-MS were represented in Table-63 & 64. Structures of the identified compounds obtained from the library of GC-MS instrument were given in Table-71 & 72.
The chemical constituents of the essential oil of Ginger rhizome were identified by the above mentioned techniques consist of 11 The GC-MS analysis showed that China ginger contained alpha-Pinene(2.337%),Bicyclo[2,2,1]heptane,2,2-dimethyl-3-methylene.(IS)-(5.94%),beta.-Phellandrene(12.00%),Eucalyptol(1.265%),2,6-Octadienal,3,7-dimethyl-,(E)-(3.91%), Benzene,1-(1,5-dimetyl-4-hexenyl)-4-methyl-(9.224%),1,6-Cyclodecadiene,1-methyl-5-methylene-8-(1-methylethyl)-,[s-(E,E]-(1.137%),1,3-Cyclohexadiene,5-(1,5-dimethyl-4-hexenyl)-methyl-,[S-R*,S*)](38.10%). alpha-Farnesene(4.573%), Cyclohexene,1-methyl-4-(5-methyl-1-methylene-4-hexenyl)-(S)-(4.391%),Cyclohexadiene,3-(1,5-dimethyl-4-hexenyl)-6-methylene-,[S-R,S)] (9.546% ),
The Chittagong ginger contained alpha-Pinene (6.630%), Bicyclo[2,2,1]heptane,2,2-dimethyl-3-methylene.(IS)-(20.60%),Cyclobutane,1,2-bis(1-methylehnyl)-,trans(2.838%), Eucalyptol (2.881%),Borneol(5.395%),2,6-Octadienal,3,7-dimethyl-,(E)-(17.90%)Benzene,1-(1,5-dimetyl-4-hexenyl)-4-methyl-(26.58%),Cycloprop[e]azulene,decahydro-1,1,7-trimethyl-4-methylene,[1aR-(1a.alpha,4a.alpha,7alpha,7a.beta,7b.alpha.)](0.854%),Cyclohexene,1-methyl-4-(5-methyl-1-methylene-4-hexenyl)-(S)-(4.99%)Cyclohexene,1,1,2tri-methyl-3,5bis(1-methylethenyl)-,(2.alpha,3.beta,5,beta.)-(1.55%), total monoterpene 56.24% & Sesquiterpene 33.97%
This was not reported earlier.The GC-MS methods which were determined in Section-4.6 The GC-MS analysis was done to ensure better identification and quantification of different components of essential oil. The percentages of various components have been determined from their peak areas. In this investigation, 12 peaks were found for 11 compounds in China Ginger sample & 12 peaks were found for 10 compounds in Chittagong Ginger sample. The major components of Zingiber officinale essential oil were found as China Ginger 1,3-Cyclohexadiene,5-(1,5-dimethyl-4-hexenyl)-methyl-,[S-R*,S*)],beta.-Phellandrene, Benzene,1-(1,5-dimetyl-4-hexenyl)-4-methyl, Cyclohexadiene,3-(1,5-dimethyl-4-hexenyl)-6-methylene-,[S-R*,S*)], .The major components of Zingiber officinale essential oil were found as Chittagong Ginger Benzene,1-(1,5-dimetyl-4-hexenyl)-4-methyl-Bicyclo[2,2,1]heptane,2,2-dimethyl-3-methylene.(IS)-,and 2,6-Octadienal,3,7-dimethyl-(E)-,total monoterpene 25.45% & Sesquiterpene 66.97%
Results show that both the two countries oils are a complex mixture of numerous compounds, many of which are found in trace amounts. It is worth mentioning that there is a great variation in the chemical composition of these two regions oil of Zingiber officinale Roscoe (Ginger) . This confirms that the reported variation in oil is due to geographic divergence and ecological conditions.
The result of the GCMS analysis report of Zingiber officinale Roscoe (Ginger) essential oil of Bangladesh and China appeared comparatively in Table-71 & 72
Table: Constituent of the essential oil of Zingiber officinale Roscoe( from , analysed by GC-MS. (Sample ID: 6601).
Peak No. | Ret. Index | % Total | Name of Compound | M.W. | M.F |
| 1 | 943
| 5.94 | Bicyclo[2,2,1]heptane,2,2-dimethyl-3-methylene.(IS) | 136 | C10H16 |
| 2 | 948 | 2.337 | alpha.- pinene | 136 | C10H16 |
| 3 | 964 | 12.00 | beta –Phellandrene | 136 | C10H16 |
| 4 | 1059 | 1.265 | Eucalyptol | 154 | C10H18O |
| 5 | 1174 | 3.91 | 2,6-Octadienal,3,7-dimethyl-,(E)- | 152 | C10H16O |
| 6 | 1446 | 9.546 | Cyclohexene,3-(1,5-dimethyl-4-hexenyl)-6-methylene-,[S-R,S)] | 204 | C15H24 |
| 7 | 1451 | 38.10 | Zingiberene | 204 | C15H24 |
| 8 | 1458 | 4.573 | alpha-Farnesene | 204 | C15H24 |
| 9 | 1500 | 4.39 | Cyclohexene,1-methyl-4-(5-methyl-1-methylene-4-hexenyl)-(S)- | 204 | C15H24 |
| 10 | 1515 | 1.137 | 1,6-Cyclodecadiene,1-methyl-5-methylene-8-(1-methylethyl)-,[s-(E,E]- | 204 | C15H24 |
| 11 | 1524 | 9.224 | Benzene,1-(1,5-dimetyl-4-hexenyl)-4-methyl | 202 | C15H22 |
| Total %
| 92.42 |
| |||
Their high concentration in rhizome, oil makes it potentially useful in medicine because they exhibit antibacterial activities. The oil has been known to be used in folk medicine in the treatment of dyspepsia, hiccough, vomiting and pain in bladder. Zingiber officinale Roscoe (Ginger) rhizome oils and extracts have been used for a wide variety of purposes for many thousands of years. In particular, the antimicrobial activity of plant oils and extracts has formed the basis of many applications, e.g. in raw and processed food 11 preservation, pharmaceuticals, alternative medicine and natural therapies.
Discussion on the fatty oil from Zingiber officinale Roscoe:
The fatty oil extracted from residue obtained after steam distillation by successive solvent extraction method using light pet-ether (40°-60°C) as an extracting solvent in a Soxh let apparatus Section-5.1
So far we aware till now the chemical composition of the fatty oil of Zingiber officinale Roscoe have not been investigated in Bangladesh by using modern analytical techniques.
Discussion on the physical characteristics of the fatty oil:
The physical characteristies such as colour, appearance, specific gravity, optical
rotation, solubility, refractive index of the fatty oil were determined by conventional methods described in Section-5.2 The result of the physical characteristics of Zingiber officinale Roscoe (Ginger) rhizome fatty oil of Bangladesh and China appeared
Comparative study of the chemical characteristics of fatty oil of Bangladeshi Ginger and China Ginger:
Chemical characteristics of the fatty oil such as acid value, ester value, saponification
value, iodine value, unsaponifable matter, peroxide value, were determined by the conventional methods
described in Section-5.6.
The result of the chemical characteristics of Zingiber officinale Roscoe (Ginger)
fatty oil of Bangladeshi Ginger and China Ginger appeared in
Table: Chemical characteristics of fatty oil:
| Chemical Characteristies | Sample collected from | |
| China Ginger | Chittagong Ginger | |
| Acid value | 12.56 | 9.6 |
| Ester value | 74.21 | 76.02 |
| Saponification value | 115.86 | 118.86 |
| Unsaponifiable matter | 34.03 | 36.06 |
| Iodine value | 78.3 | 80.56 |
| Peroxide value | 31.46 | 32.62 |
Discussion on mineral elemental Analysis of Zingiber officinale Roscoe (Ginger):
The mineral content was determined by AAS (Atomic absorption Spectrometer). Model no: Rigaku Primus ZSX, made by USA. Instrument type: wave length dispersive AAS (WDXRF) with SC and PC type detector, AAS equipped with an X-ray tube anode Rh tube, and also five filters, the AAS instrument was fully” automatic. And P10 gas was used and mineral element concentration was detected on dry matter basis. Which have described in Section-6.3
Mineral contents of Zingiber officinale Roscoe (ginger):
Mineral content such as Na, Mg, P, S, K, Ca, Cr, Mn, Fe, Ni, B, Pb, Cd etc. were determined in its fresh ginger powder. Ginger rhizomes were collected from Bangladesh and China. The result of element analysis of its rhizome was determined by AAS(Atomic absorption Spectrometry )method in g/l00g dry weight basis of the sample. The element analysis of Zingiber officinale Roscoe (ginger) rhizome can be compared with different countries. The result of minerals content of ginger rhizome of Bangladesh and china appeared as element basis in Table-75
The total concentration of macro and.micro elements were measured in ginger rhizome from two different places of Bangladesh and China by AAS (Atomic Absorption spectrometry) for the first time in Bangladesh by the authors. Precision of the measurements was taken by analysis of two samples of Bangladesh and China.
Table-: Elemental analysis in percent comparatively (on dry matter basis) of Zingiber officinale Roscoe (Ginger) rhizome of Bgladesh and China (g/l00g).
No. | Component as Element | Name of the region | |
China Ginger | Chittagong Ginger | ||
1 | Na | 0.34 % | 0.24 % |
2 | Mg | 0.809 % | 0.611 % |
3 | P | 1.12 % | 1.20 % |
4 | S | 0.130 % | 0.050 % |
5 | K | 0.68 % | 0.244 % |
6 | Ca | 2.43 % | 1.70 % |
7 | Mn | 15.51µg/L | 17.26µg/L |
8 | Fe | 2.48µg/L | 3.69µg/L |
9 | Ni | 3.0µg/L | 1.70µg/L |
10 | B | 40.0µg/L | 35µg/L |
11 | Cd | 1.20µg/L | 1.10µg/L |
12 | Pb | 1.90µg/L | 3.50µg/L |
13 | Cr | Trace | Trace |
China ginger total percent of minerals 69.599 & Chittangong ginger total percent of minerals 66.295.
In our study some variation is observed in our data. These variation may be due to on such factors as type of genetic variety, maturity, collection time, climatic condition in geographical location, composition of the soil, water, fertilizer used as well as permissibility, selectivity and absorbility of plants for the uptake of these parameter. All the effects caused the final level of mineral components in a rhizome.
So far we aware till now the elemental composition of Zingiber officinale Roscoe (Ginger) rhizome have not been investigated on elemental analysis in Bangladesh by using modern analytical techniques such as AAS analysis anywhere and were not reported earlier.
Results and Discussion of Antimicrobial Activity
Ginger essential oil of PE, DCM, EA & ME (100, 200, 400 μg/disc) were screened against 13 test bacteria (5 gram positive and 8 gram negative organisms and 3 fungi). In the dose of 100 μg/disc, the ME extract showed the strongest inhibitory activity against Vibrio mimicus, Staphylococcus aureus & Sarcina lutea having the zone size 13 mm. The growth of Bacillus cereus, Salmonella paratyphi, Escherichia coli, Sacharomyces cerevaceae & Aspergillus niger were moderate (12 mm) inhibited in the ME extract. Mild activity were found (10 mm zone inhibition of each) in the same extract against Bacillus megaterium, Salmonella typhi , Shigella dysenteriae, Shigella boydii & Candida albicans . At the same time, the DCM extract showed mild inhibitory activity against the entire organism having zone of inhibition 7-9 mm. The PE extract inhibited the very low growth having zone of inhibition 7 mm except Escherichia coli (8mm). The EA extracts showed very weak antimicrobial activity.
Bacillus megaterium, Bacillus subtilis & Salmonella paratyphi showed zone of inhibition (7mm of each) and rest of the micro organism were insensitive to EA extract. In 100 μg/disc, the DCM, PE and EA extracts are insensitive to the all tested micro organism except ME extract. On the other hand 200 μg/disc, the PE & EA extracts are insensitive to all the micro organism and ME & DCM extracts are very low sensitive some of the organism. At the dose of 400 μg/disc, the compound AP-10 (from ME extract) showed highly sensitive to Bacillus megaterium , Escherichia coli , Candida albicans, Aspergillus niger & Sacharomyces cerevaceae having the zone size 12mm and also showed moderate sensitive to rest of the organism. At the dose of 100 & 200 μg/disc most of the organisms are sensitive where Bacillus megaterium and Bacillus subtilis are insensitive at that dose level. Sarcina lutea, Escherichia coli, Shigella boydii, Candida albicans & Sacharomyces cerevaceae are insensitive at 100 μg/disc dose level. Moreover, the ME extract and its compound AP-10 have significant activity against microorganisms and they can be used in the treatment of infectious diseases caused by microbes.
Results and Discussion of Antioxidant Activity
The average IC50 value of ASA 5.85, the average IC50 value of BHT 28.37,
The average IC50 value of China ginger essential oil 61.25, the average IC50 value of Chittagong ginger 56.71.
Results and Discussion of cytotoxic activity
EESD showed cytotoxic activity against brine shrimp & the LC50 value found 40.39µg/ml compared to vineristine sulphate 0.8094 µg/ml
Conclusion
At present, it is estimated that about 80% of the would population relies on botanical preparations as medicines to meet their health needs. Herbs and species are generally considered safe and proved to be effective against curtain ailments. Ginger is mostly.
Ginger is mostly used for the purpose of manufacturing various types of medicine. Ginger is well known as a remedy for travel sickness, nausea and indigestion and is used for wind, colic, irritable bowel, loss of appetite, chills, cold, flu, poor circulation, menstrual cramps, dyspepsia (bloating, heartburn, flatulence), indigestion and gastrointestianl problems such as gas and stomach cramps. Ginger is powerful anti-inflammatory herb and there has been much recent interest in its use for joint problems. It has also been indicated for arthritis, fevers, headaches, toothaches, coughs, bronchitis, osteoarthritis, rheumatoid arthritis, to ease tendonitis, lower cholesterol and blood-pressure and aid in preventing internal blood clots.
The Medicinal value of parts lives in some chemical substances that produce a definite physiologic action on the human body. The most important forms of spice extracts are oleoresins and volatile oils or essential oils. Essential oils are volatile liquid with strong odor. They contain complex compounds, formed by aromatic plants of secondary metabolites. Essential oils of some spices are utilized in food industry Preservatives and in perfumery and medical Industries.It also activites work as antimicrobial, antixodamt and cytotoxicity.