Phytochemical and Biological Evaluation of
Albizia richadiana (Benth. Fabaceae family)
This dissertation aimed at to investigate and evaluate the possible phytochemical and biological profiles like, phytochemical screening, brine shrimp lethality bioassay and hypoglycemic activity bof the medicinal plant Albizia richardiana (Fam. Fabaceae, subfam. Mimosoideae). Plants of the Albizia species are employed as traditional medicine for the treatment of anthelmentic, rheumatism, cough, diarrhea, stomach ache, wounds, etc. These plants are also recognized therapeutically for treating irritability, insomnia, wounds, as antidysentric, antiseptic, antitubercular etc. In present Phytochemical study, this plant is a great source of different group of bioactive chemical substances like- carbohydrates, saponins, glucosides, glycosides and alkaloids. Apart from this, they may show cytotoxicity as well as hyperglycemic activity. Based on the present study, we can suggest Albizia richardiana may be given the recognition as a medicinal plant.
Introduction
Staying healthy, free from diseases has always been people‟s prime concern since the very beginning of their existence on this earth. In an effort to accomplish this concern, human being had utilized and also made attempts to use any materials they thought to be useful in their struggle of existence.
It is always observed that natural propensity of human being is to avoid illness, physical discomfort, injuries, wounds and obviously death. They tried many things and at the end they follow nature. They found out plants with pharmacological and medicinal properties. However, early men didn‟t know the actual uses or pharmacological properties of those plants; they selected those plants based on trials and errors. For relieving pain and suffering caused by abnormal conditions and also for preserving health against disease and death, people became familiar with several plants containing particular chemical compounds that can be used for treating particular illness or discomfort or diseases. Therefore, there has always been an effort to discover new chemical entity that can be used for treating particular disease. (Ghani.A. 2003)
However, it is seen from the ancient period, medicinal plants have been comprehensively used for medical purposes throughout all generation and all the prehistoric society had contributed significantly for enriching the knowledge of medicinal uses of plants by mentioning the remedies for diverse diseases whether it is simple wound and skin diseases or more complicated formidable heart diseases and cancer etc. (Ghani.A. 2003)
A short history of the uses of medicinal plants is given in the following part.
- In 3000 BC: It is observed from different studies that a large number of medicinal plants were used by the Babylonians. Of course they were aware of their properties. Nevertheless, early people like Assyrians, Babylonians, Egyptians and ancient Hebrew, were also recognized for knowing the properties of many medicinal plants. However, the use of some medicinal plants that were used by Babylon still exists today with same manner and purpose. (Ghani.A. 2003)
- In 4500-1600 BC: Huge information about the uses of medicinal plants in the Indian subcontinent during this period is mentioned in Rig Veda, the oldest book in library of man. For example, the use of Soma plant (Amanita muscaria) which is used as a medicinal agent as narcotic and hallucinogenic agent and Sarpagondha (Rauvolfia serpentina) which is used as healing plant are mentioned in this book. Susruta Samhita and Charaka Samhita are the two other oldest book from which information about the Indian medical systems and medicinal plants can be obtained. (Ghani.A. 2003)
- In 1500 BC: Papyrus Ebers, contains a good knowledge about the properties of hundred of medicinal plants. It is also seen from different observations that today‟s most important plants, were also used in Egypt about 4500 years ago (Hill, 1972), such ashenbane (Hyoscyamusspp.), mandrake (Mandragoraofficinarum), opium (latex of the mature but unripe fruits of Papaversomniferum), pomegranate (fruits of Punicagranatum), castor oil (oil of Ricinuscommunis seed), aloe (juice of leaves of Aloe spp.), onion (Allium cepa), hemlock (Conium spp.), cannabis (Cannabis sativa), senna (Cassia senna) and many others. However, since they had the vast knowledge about the properties of medicinal plants and human anatomy, they use plant parts and extracts also for the preservation of dead bodies as mummy, (Ghani.A. 2003)
- In 1200 BC: Ayurvedic system described the medicinal uses of about 127 plants (Obianwu, 1984).
After 131 AD: Galen (131-200 AD), the great Greek pharmacist-physician, was the first to include the procedures and methods for preparing therapeutic recipes. He attempted to include ingredients of both plant and animal origins (Claus & Tyler, Jr., 1965). He wrote about 500 volumes of books, describing a large number of medicinal plants and animal products with hundreds of recipes and formulations. (Ghani.A. 2003)
But, before going to further discussion, one must be very much clear about the medicinal plants. Medicinal plants are plants with therapeutic properties and may use for beneficial pharmacological effects on animal body. These plants are not morphologically different from other plants but may have various special qualities which make them medicinally important than others. However, it is observed from different studies, plants that are naturally synthesized and contain secondary metabolites, more instance alkaloids, sterols, terpenes, flavonoids, saponins, glycosides, cyanogenics, tannins, resins, lactones, quinones, volatile oils, etc as well as minerals Phytochemical and Biological Evaluation of Albizia richardiana benth. Fabaceae family 3 and vitamins, may have medicinal properties. With this continuation, World Health Organization (WHO) consultative group had given a definition on medicinal plants in the following way: “A medicinal plant is any plant which, in one or more of its organs, contains substances that can be used for therapeutic purposes or which are precursors for synthesis ofuseful drugs.” (Sofowora, 1982).”
However, early man didn‟t have any special knowledge about medicinal plants. They discovered plants with medicinal properties not only from their intuition, guesswork or trial and error approach, but also from their curiosity and searching trend for food. Sequentially they gather knowledge about medicinal plants. However, now-a-days, with the advancement of technology, people are using modern techniques to discover new drugs. For this reason, they are working on different unknown plants for enlisting them to the list of medicinal plants.
In country like Bangladesh, there are about 6500 plant species which may include plants like bryophytes, pteridophytes, gymnosperms, and angiosperms. This large diverse plant features is available in the country due to its favorable agroclimatic circumstance with productive soils, a tropical climate, and seasonal diversity. However, among the large diversity, about 500 plant species contain medicinal values. These medicinal plants are normally found to grow as native, naturally occurring, or cultivated plants in the country‟s forests, wetlands, homestead forests, and also even roadside areas. However, in country like Bangladesh, around 75% (10 million households in over 85,000 villages) of the country‟s total population lives in rural areas and almost 80% of them are reliant on natural resources (e.g., medicinal plants) for their primary healthcare as herbal medication.
Herbal medication represents a well known and accepted form of treatment among the rural people in Bangladesh. This is may be due to the capability of those people to recognize many species of plants in their areas from which various products like food, firewood, medicine, forage, etc. can be obtained. Apart from this, the traditional homestead tree production system serves as a source of plant products and remedies in the country (Rahman M.H., 2013). But it is quite unfortunate for us that between the 25 000 and 75 000 plant species that are used as traditional medicine, only 1% are known by scientists and accepted for commercial purposes (Aguilar, 2001). Nevertheless, to meet the increasing demand, about 500 companies are established in the country, but still more are needed to fulfill the demand of herbal medicine. For Phytochemical and Biological Evaluation of Albizia richardiana benth. Fabaceae family 4 this purpose, more research are needed to be conducted so that we can compete with this neverending require. (Ghani.A. 2003)
The work elucidated in this dissertation is an attempt to evaluate the possible biological profiles of the medicinal plant Albizia richardiana (Fam. Fabaceae, subfam. Mimosoideae). The genus of the plant is Albizia. The other plants of this genus have various significant medicinal properties for instance they are recognized to treat asthma, arthritis, antiseptic, burns, antidysentric, allergic rhinitis etc. But no significant amount of work has been done on the plant Albizia richardiana of this genus. So the main objective of our dissertation is:
To find out and evaluate the phytochemical and biological profiles of the medicinal plant Albizia richardian, so that it can be given the recognition of medicinal plant.
The plant: Albizia richardiana
The plant under investigation, Albizia richardiana is locally known as Raj koroi and the synonym for the plant name is Albizia niopoides. The plant belongs to the family Fabaceae and the subfamily Mimosaceae. However, the plant is also known as Albizia paludosa. The plant is generally recognized as the ornamental plant in tropical areas of USDA Zones 10 and above. It is a fast growing plant, normally found in Bangladesh (Das and Alam, 2001). The species is usually found to grow in completely hot and humid environment like Asia, Africa, Madagascar, North America and Australia, but mostly in the old world tropics (Azad et al. 2010). In Bangladesh, this species is usually found to grow in the forest of Sunamgonj, in the forest of Chittagong Hill Tracts (Kaptai) and also in the village of northern districts. (Rahman. M. and Kumar Das.A.2014)
Albizia richardiana is a large, dry tree with contrastive characteristics like temporary and quick growing nature. It contains an open, spreading, beautiful, light green, very finely pinnate and crown like leaves and powder-puff like flowers. (Rahman. M. and Kumar Das.A.2014) Considering the usage of this plant, this plant is known as an ornamental plant in general.
However, it is an important component of the village and society of our country as being an important part of wood based industry. In Bangladesh, the timber of Albizia richardiana is Phytochemical and Biological Evaluation of Albizia richardiana benth. Fabaceae family 5 generally used for construction purposes. However, medicinal use of this plant has not been extensively studied. (Rahman. M. and Kumar Das.A.2014)
General botanic data
The plant is an anise. It is a fast growing tree with pseudo dichotomously branching habit and straight bole, which can form a beautiful crown. Structural features of the plant are given below:
Leaves: Leaves are bipinnated compound. Rachis are about 12 cm long, channeled above, usually with a cup-shaped gland on the petioles near the base and 1-3 small similar glands between the bases of distal pairs of pinnae, usually 8-14 pairs, 4.5-7.5 cm long with pulverous base, softly white tomentose along the upper sides, leaflets 60-100 pairs, 6 x 1 mm, linearfalcate, sessile, acute, entire, base unequal, lower side auricled, midrid closer to upper margin, glabrous, smooth and dark green leaflets in very close set. Inflorescence axillary or terminal panicles are shorter than the leaves, in short pedunculate, globose heads, and peduncles. Usually 1.0-1.4 cm long and head many flowered. The tree sheds leaves at the end of winter and leaves are replaced during early summer.
Flowers: Flowers are small, greenish white and sessile. Calyx is funnel-shaped, tomentose from outside, and in very minute amount. Corollas are campanulated with 5 lobes, oblong, lanceolated and acute. Stamens contain 25-30, filiform, exserted and small anthers. Ovaries are directly attached to the stem.
Fruit: Fruit contains a pod, 8-10 x 2.0-2.3 cm, and thin firm, flat and strap-shaped, shortly beaked, dull grayish brown. Seeds: Seeds are 8-12 per pod, and 6-8 mm long, oblique towards the tip near attachment with the funicle. Funicle is yellow in color and as long as seed. Flowering and fruiting time: The flowering and fruiting time period is between August December of the year.

The plant family: Fabaceae
The name ‘Fabaceae’, which is now included in Vicia, comes from the extinct genus faba. The term “faba” is a Latin word and represents simply “bean”. Leguminosae is an older validated name, comes from its fruits name which are called legumes.
The Fabaceae or Leguminosae family is also known as legume, pea, or bean family. This large family is usually known for economical importance because of flowering plants. However, the family is rich with trees, shrubs and herbaceous plants. The plants of this family are either annuals or perennials. They can be easily identified by their fruit (legume) and specified leaves.
According to the number of species, the family is the third-largest land plant family and extensively distributed. This family contains about 630 genera and over 18,860 species. They are commonly found in the tropical rainforests as well as in the dry forests of Americas and Africa.
The history of this family is closely related to the human evolution, since along being used as cereals. Their some fruits and tropical roots in a few number of Leguminosae have been used as staple human food for millennia.
However, Fabaceae is a single monophyletic family, which is established by the molecular and morphological evidences. It is also supported by both the degree of interrelation of different groups within the family and phytoogenetic studies based on DNA sequences. These studies has also confirmed that Leguminosae is closely associated with Polygalaceae, Surianaceae and Quillajaceae families which are belonging to the order Fabales, a monophyletic group.
The family is rich with a number of significant agricultural and food plants, which include Glycine max (soybean), Phaseolus (bean), Pisum sativum (pea), Cicer arietinum (chickpeas), Medicago sativa (alfalfa), Arachis hypogaea (peanut), Lathyrus odoratus (sweetpea), Ceratonia siliqua (carbo) and Glycyrrhiza glabra (liquorice). A number of species are also found as weedy pests in different parts of the world, which includes Cytisus scoparius (broom), Robinia pseudoacacia (black locust), Ulex europaeus (gorse) and Pueraria lobata (kudzu).
Some of the features of this family are given below:
Distribution and habitat:
The Fabaceae have been found almost everywhere except Antarctica and the high arctic. Whereas, the herbaceous plants and shrubs mainly predominant in extratropical areas, with main occurrence in tropical regions.
Description:
The plant of Fabaceae family range from enormous trees (like Koompassia excelsa) to minute annual herbs, with the greater part of the plants being perennials. Plants, which contain imprecise inflorescences, occasionally reduced to a single flower. The flowers, which have a short hypanthium and a solitary carpel with a short gynophore, produce fruits after fertilization called legumes.
Growth habit:
The Leguminosae have a wide diversity of growth forms which includes trees, shrubs or herbaceous plants and even vines or lianas. This type of plants can be annuals, biennials or perennials. The plants can be without basal or terminal leaf aggregations. They are vertical plants, epiphytes or vines. The latter support themselves by means of shoots which twist around a support or through cauline or foliar tendrils. However, these plants can be heliophytes, mesophytes or xerophytes.
Plant Physiology:
Leaves: The leaves are generally alternate and compound. Frequently they are even and odd pinnate (e.g. Caragana and Robinia respectively), often trifoliate (e.g. Trifolium,Medicago) and seldom palmately compound (e.g. Lupinus). But in the Mimosoideae and the Caesalpinioideae subfamilies, they are usually bipinnate (e.g. Acacia, Mimosa). They always have stipules, which can be leaf-like (e.g. Pisum), thorn-like (e.g. Robinia) or can be rather unremarkable. Leaf borders are entirely or, rarely serrate. Both the leaves and the leaflets generally have wrinkled pulvini to allow nastic movements. However, in a few species, leaflets have developed into tendrils (e.g. Vicia).
It is also observed that in various species, they have leaves with structures which magnetize the ants which may protect the plants from herbivore insects (a form of mutualism). It is also observed that extrafloralnectaries are familiar among the Mimosoideae and the Caesalpinioideae, and may also be found in several Faboideae (e.g. Vicia sativa). However, in a few Acacia, the customized hollow stipules are inhabited by ants, known as domatia.
Roots: Various Fabaceae host bacteria in their roots, called root nodules. These bacteria, known as rhizobia, contain the capability to take nitrogen gas (N2) out of the air and convert it to a form of nitrogen, which is then further used by the host plant (NO3or NH3 ). The process is known as nitrogen fixation. In this way, the legume, acting as a host, and rhizobia, acting as a provider of usable nitrate, form a symbiotic relationship. Phytochemical and Biological Evaluation of Albizia richardiana benth. Fabaceae family 11
Flowers: The flowers of this family usually contain five fused sepals and five free petals. Generally they are hermaphrodite. The flowers contain a petite hypanthium, frequently cup shaped. There are also ten stamens and one extended superior ovary which occur in curved style. They are arranged in indeterminate inflorescences. Fabaceae are characteristically entomophilous plants (i.e. they are pollinated by insects), and their flowers are habitually showy to catch the attention of pollinators. In the Caesalpinioideae, the flowers are repeatedly either zygomorphic, as in Cercis, or almost symmetrical with five identical petals as in Bauhinia. The upper petal is the deepest one, except in Faboideae. A few species, like some in genus Senna, include asymmetric flowers and this style twisted to one side. The calyx, corolla, or stamens can also be showy in this group.
In the Mimosoideae, flowers are actinomorphic and arranged in globose inflorescences. The showiest part of the flower is petals, which are minute and the stamens, which can be more than just 10, have long, coloured filaments. However, all the flowers open at once.
In the Faboideae, the flowers are zygomorphic, and also contain a specialized structure. The upper petal is large and encloses, called the banner and the remaining petals often reflexing in bud when the flower blooms. The two adjacent petals, called the wings, surround the two bottom petals. These two bottom petals are fused together at the apex (remaining free at the base) and forming a boat-like structure called the keel. The stamens are always ten in number, can be often in a group of nine stamens plus one separate stamen. The filaments of stamens can be fused in various configurations.
Fruit: Usually the ovary builds up into a legume. A simple dry fruit like legume habitually dehisces (opens along a seam) on two sides. They have a common name called “pod”. This name can also be utilized to a few other fruit types. From the basic legume fruit a small number of species have developed for instance samarae, loments, follicles, indehiscent legumes, achenes, drupes, and berries.
Plant biochemistry:
Sometimes the Leguminosae are cyanogenic and their cyanogenocity is derived from tyrosine, phenylalanine or leucine, and frequent presence of alkaloids in them. Proanthocyanidins also be able to present either as cyaniding or delphinidine or both at the same time. As well as Flavonoids like kaempferol, quercitin and myricetin are often found to be present. In any of the genera or species, which are analyzed, Ellagic acid has never been found.
In the form of sucrose, sugars are transported within the plants. C3 photosynthesis has also been found in a wide variety of genera. However, Pterocarpans (derivatives of isoflavonoids) are found only in the Fabaceae.
Pharmacological properties of the family:
Various plants like Albizia lebbeck, Albizia julibrissin, Albizia saman, Albizia inundata, Albizia odoratissima, Albizia mollis, Albizia procera, Albizia gummifera, Albizia amara, Albizia schimperiana are included in this family. All those plants contain different kinds of pharmacological properties.
Extract preparation of Albizia richardiana
Collection and Preparation of the Plant material
Bark of Albizia richardiana was collected from the Chittagong hill area, Bangladesh, as plant sample. The sample was then identified by an expert taxonomist at S.K.H.D.U (Acc. No.10778).
Then it was cut into small pieces for better drying as we know evaporation increases with the incensement of the surface area. After that it was sun dried for several days which is then followed by oven drying. The dried bark was then crushed into coarse powder by a high capacity grinding machine, which was well cleaned before grinding to avoid cross contamination.
Extraction of the Plant material
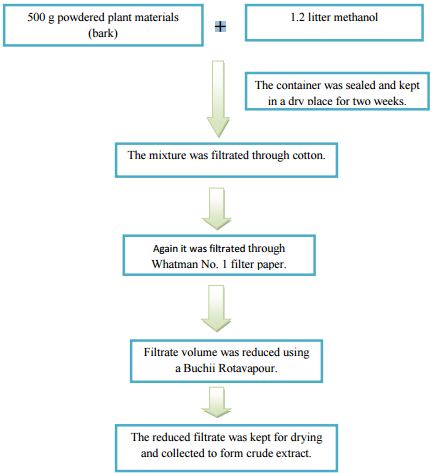
Two amber containers (2.5) liters were used for soaking 500 g powdered plant materials (bark) in 1.2 liter of methanol as solvent. These two containers together with its contents were sealed by cotton plug and aluminum foil. Then these two containers were reserved like this in a dry place for about a period of two weeks with occasional shaking and stirring. After two weeks whole mixture was filtered, at first through cotton and after that through Whatman No. 1 filter paper.
From this filtration, about 525ml filtrate was obtained. The filtrate volume was then reduced using a Buchii Rotavapour at low temperature and pressure, after which, the final volume of the filtrate was 100 ml. Then the filtrate was kept in a beaker and sealed with aluminum foil with Phytochemical and Biological Evaluation of Albizia richardiana benth. Fabaceae family 18 several pores on it. After that the filtrate was kept for drying to become crude dry extract. The weight of final extract was 12 g +.
Design of Biological Investigations
One of the successful sources of drugs is Natural products. Based on “rational” drug design, many new chemotherapeutic agents are synthetically derived. The natural products have advantage above synthetic or semi-synthetic drug and that leads optimally to materials containing new structural features with significant biological activities. The drugs which are used now-a-days come from natural sources. Practically every pharmacological group of drugs may include a natural product prototype. As sources of plants for the use of analysis, preclusion, and treatment of diseases is very promising (Setzer W.N., 2000).
Natural products are mainly metabolites, which can be derived naturally and/or can be the byproducts from microorganisms, plants, or animals. Structural diversity is the major advantages of natural products for random screening. It is achievable to isolate a number of homologues and obtain structure- activity relationship, wherever bioactive natural products take place as a part of a family of related molecules. Lead compounds can be optimized either by traditional medicinal chemistry or by application of combinatorial approaches which are found from screening of natural products (Wikipedia 2007). Overall, natural products library, seems more likely to make available the chemical diversity to yield a hit than a library or similar number of compounds made by combinatorial synthesis, is used in case of screening assays where is present no information about low molecular weight leads. Since, for biological activities only a small portion of the worlds‟ biodiversity has been examined and it can be assumed that natural products will continue to offer novel leads for novel therapeutic agents.
Experimental Design
Investigation of chemical compounds by phytochemical screening
The methanolic bark extract of Albizia richardiana was subjected to analysis chemical screening for the identification of the presence of bioactive substances like, carbohydrate, tannin, glycosides, alkaloids, and saponins etc. by using standard procedures.
Brine Shrimp lethality Bioassay
For the screening of pharmacological activities in plant extracts, Brine shrimp lethality bioassay (Mayer et al., 1986) has been suggested. Rapid, inexpensive and simplicity (eg.no aseptic techniques are required) are the advantages of this method. For statistical validation, this method easily utilizes a large number of organisms and requires no special equipment and a relatively small amount of sample. Additionally, it does not require animal serum.
Evaluation of hypoglycemic activity
For the evaluation of hypoglycemic activity, the most acceptable method is glucose tolerance test (GTT). In this medical test, glucose is given and blood samples are taken subsequently to find out how quickly the glucose is cleared from blood. The test is usually used for the purpose of determining diabetes, insulin resistance, and occasionally reactive hypoglycemia or rare disorder of carbohydrate metabolism. Many variation and development of the GTT have been done over the years for various purposes, with different standard doses of glucose, different routes of administration, different intervals and durations of sampling with the measurement of various substances in addition to blood glucose.
Investigation of Chemical Compounds by Phytochemical Screening
Introduction
For the preliminary analysis and identification of nature and quantity of bioactive chemical substances present in a given plant material, phytochemical screening can act as an important and essential means of analysis.
For the present investigation, the methanolic bark extract of Albizia richardiana was subjected for phytochemical analysis to identify the presence of bioactive substances like, carbohydrate, tannin, glycosides, alkaloids, and saponins etc. by using standard procedures.
Methods of the experiment
A large variety of qualitative tests are available for the phytochemical screening of medicinal and other plants. There are some common standard tests described here which are simple, rapid and available for such phytochemical screening.
Test for Carbohydrates
- Molisch’s test: At first, a test tube was taken and 2 mL of plant‟s aqueous extract was poured into it, then 2 drops of freshly prepared 10% of alcoholic solution of alpha naphthol was added in the test tube and mixed thoroughly. After that, 2 mL of conc. sulphuric acid was allowed to flow down the side of the inclined test tube so that the acid forms a layer beneath the aqueous solution. If carbohydrate is present, a red or reddish violet ring is formed at the junction of the two layers, which is followed by formation of a dark purple solution on standing or shaking. Then, it was shaken followed by standing for about two minutes. After that, the solution was diluted with 5 mL of water. A dull violet precipitate formed immediately will indicate the presence of carbohydrate in the plant material.
- Fehling’s test: For doing the Fehling‟s test, 1 mL of a solution, which was the mixture of Fehling’s solutions A and B (equal in volume), was added to the 2 mL volume of the methanolic extract of the plant material. After that the mixture was boiled for a few minutes. Formation of a red or brick-red precipitate will indicate the presence of reducing sugar in plant extract.
Test for Combined Reducing Sugar
About 1 mL of plant‟s aqueous extract and 2 mL of dilute hydrochloric acid were boiled together for 5 minutes. Then the mixture was cooled followed by neutralization by using sodium hydroxide solution. At the end, Fehling‟s test was carried out. A combined reducing sugar is present (due to the release of the reducing sugars on hydrolysis), if a brick-red precipitate is formed.
Tests for Glycosides
For doing this test, a small quantity of an alcoholic extract of the fresh and dried plant material was dissolved in about 1 mL of water. After that a few drops of aqueous sodium hydroxide solution were added to it. If the plant material contains glycosides, a yellow color may develop.
Test for Glucosides
A small quantity of plant‟s alcoholic extract was allowed to dissolve using both water and alcohol. After that, the solution was divided into two portions and was treated in the following ways:
- First portion was boiled up with solution containing equal volumes of Fehling’s solutions A and B and note was taken whether any brick-red precipitate was formed or not.
- Other portion was boiled for about 5 minutes with a few drops of dilute sulphuric acid. After that, the mixture was neutralized by using sodium hydroxide solution. Then a solution containing equal quantity of Fehling‟s solutions A and B, was added to it followed by boiling the mixture again.
The second experiment was carried out with the hydrolysed extract. Formation of a brick-red precipitate and formation of no such precipitate in first experiment may suggest the presence of glucosides in the extract.
Tests for Saponins
- About 0.1 g of the powdered plant material was allowed to be dissolved in about 10 mL of water. Then the mixture was allowed to be boiled for about 3-5 minutes. Then the mixture was filtered. After cooling, about 5 mL of the filtrate was diluted with water and then shaken vigorously. Formations of persistent frothing, which remain stable on heating, indicate the presence of saponins.
- Alternatively, this test can be performed by shaking 0.5 g of plant‟s alcoholic extract with water. A similar result can be obtained.
- Tests for Flavonoids
- A small quantity of plant‟s alcoholic extract was taken and a few drops of conc. hydrochloric acid were added into it. Formation of red color immediately after the addition may suggest the presence of flavonoids in the plant material.
- A test tube was taken and about 0.5 mL of an alcoholic extract of the sample was poured into it. Then about 5-10 drops of conc. hydrochloric acid with a small piece of magnesium or zinc ribbon was added to it. Then the mixture was boiled for about few minutes. The result will be based on the development of the following color change likeorange to red (flavanes), red to crimson (flavanols), crimson to magenta (flavanones) and occasionally crimson to green or blue color (flavonoids).
Test for Tannins
Ferric chloride test: About 5-10 mL of distilled water and 0.5 g of either an alcoholic or aqueous extract of the plant material were taken. Then the extract was dissolved in water after which it was filtrated. After that, a few drops of 5% ferric chloride solution was added to the filtrate. Formation of blue, blue-black, green or blue-green colors precipitate may indicate the presence of tannins. On the addition of a few mL of dilute sulphuric acid, this color may disappear and may form a yellowish brown precipitate.
Test for Resins
A small quantity of chloroformic or ethanolic extract was dissolved in about 5 to 10 mL of acetic anhydride by using gentle heat. After that, the solution was cooled and then about 0.05 mL of sulphuric acid was added to it. A bright purplish red color, rapidly changing to violet, may form if resin is present in the plant extract.
Tests for Proteins
- Millon‟s test: About 5-6 drops of Millon‟s reagent was added to a solution containing a small quantity of methanolic extract of the plant material which dissolved in about 1 mL of distilled water. On heating, if a white precipitate turns into red colour, it may suggest the presence of protein in the sample.
- Biuret‟s test: About 5-8 drops of 10% sodium hydroxide solution and 1-2 drops of 3% copper sulphate solution were added to 1 mL of a hot aqueous extract of the plant material. A red or violet color may produce if protein is present.
Test for Alkaloids
Alkaloid extract does not always give satisfactory results while using organic solvents or even
aqueous organic solvents directly, though alkaloids occur in plants both as free bases and as their salts. In this case, extraction of the alkaloidal constituents can be affected by using either acidified dilute alcohol or pretreatment of the plant material with dilute ammonia solution or calcium hydroxide with sufficient water. This is performed to moisten the powdered plant material prior to extraction with organic solvents. Extraction of the material can be completed by either maceration or percolation or by the use of Soxhlet extractor.
General laboratory test: About 0.5 g of the extract was added to 5 mL of 1% hydrochloric acid and then the mixture was put on a steam bath, after which it was filtered. Then about 1 mL of the filtrate was treated with a few drops of each of the following reagents, separately. Turbidity or formation of the respective colored precipitates may indicate the presence of alkaloids in the extract:
- Mayer’s reagent (potassio-mercuric iodide solution): In presence of alkaloids, this reagent will produce white or creamy white precipitate.
- Hager’s reagent (1% solution of picric acid): In presence of alkaloids this reagent will produce yellow crystalline precipitate.
- Wagner’s reagent (saturated solution of I2 in KI): In presence of alkaloids this reagent will produce brown or deep brown precipitate.
- Dragendorff’s reagent (bismuth potassium iodide solution): In presence of alkaloids this reagent will produce orange or orange-red precipitate.
- Tannic acid solution (10%): In presence of alkaloids this reagent will produce dirty white or black precipitate.
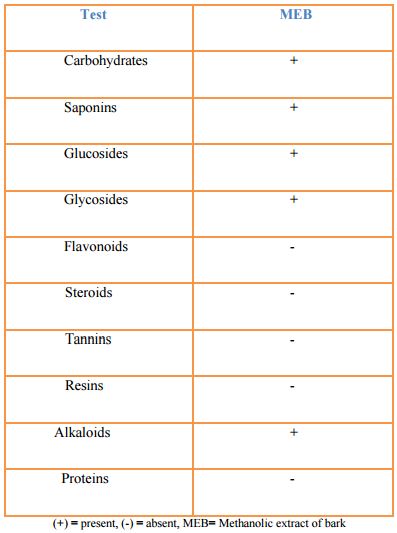
Results and discussion
Phytochemical screening test of the methanolic plant extract of Albizia richardiana suggests the presence of bioactive substances like- carbohydrates, saponin, glycosides, glucosides, alkaloids in the bark extract of plant. Results of phytochemical screening of Albizia richardiana is given in the following table.
Table: Result of phytochemical screening
Brine shrimp lethality bioassay
Introduction
For the evaluation of cytotoxicity, the bioactive compounds of natural and synthetic origins are assayed by Brine Shrimp Lethality bioassay. This is a rapid and comprehensive bioassay method.
In the discovery of new bioactive natural products, the method utilizes in vivo lethality in a simple zoological organism (Brine nauplii) which is a convenient monitor for screening cytotoxic activity. Brine toxicity is closely associated to 9KB (human nasopharyngeal carcinoma) cytotoxity (p=0.036 and kappa = 0.56). However, the assay contains some advantages for being rapid (24 hours), inexpensive, and simple (e.g., no aseptic technique are required). For the purpose of statistical validation, a large number of organisms can also easily be utilized by this method. Apart from this the method requires relatively small quantity of sample (2-20 mg or less) with no special equipment. Additionally, no animal serum is needed for cytotoxic activity evaluation.
Materials
- Artemia salina leach (brine shrimp eggs)
- Sea salt (NaCl)
- Small tank with perforated dividing dam to hatch the shrimp
- Lamp to attract shrimps
- Micropipette (100-1000 µl)
- Glass vials
- Magnifying glass
- Test samples of experimental plants
Experimental Procedure
In brine shrimp bioassay method, brine shrimp eggs were hatched in simulated sea water. Then different calculated amount of methanolic extract of bark of Albizia richardiana were dissolved in dimethylsulphoxide (DMSO) solvent and preferred concentration of the test sample were prepared. Then, the nauplii were counted by visual inspection after which the nauplii were transferred in vials containing 5 mL of simulated sea water. By using micropipette, samples of different concentrations were added to the premarked vials. The vials were then left for 24 hours and maintained in required conditions. Counting of the Survivors took place after 24 hours.
Preparation of seawater
For hatching of brine shrimp, simulated seawater was needed. For that purpose, 38g of sea salt (pure NaCl) was dissolved in 1 liter of distilled water. At first, 38g of sea salt (pure NaCl) was weighed by rough balance and dissolved in one liter of distilled water, in a small tank and after which it was filtered to obtain a clear solution.
Hatching of brine shrimps
Artemia saline leacheds (Brine Shrimp eggs) were collected from nearby pet shops was used as test organism. The quantity of simulated seawater was prepared according to the size of the tank, as the simulated seawater was poured down in a small tank. For this test, 2 liter of simulated seawater were prepared and then Shrimp eggs were added to the one side of the perforated divided tank and after which the side was covered.
The tank was kept under constant aeration for 48 hrs for hatching the shrimp and to be matured as nauplii (Iarvae). Then 10 living Shrimps were added to each of the test tubes, which containing 5 ml of Brine solution. All through the hatching time constant oxygen supply had to carry out, by using pump. The hatched shrimps were attracted to the lamp through the perforated dam and they were taken for experiment. 10 living shrimps were added to each of the test tubes, which containing 5 mL of seawater. This work is done by getting the help from a Pasteur pipette.
Preparation of Test Solutions
About 5 mg of the plant‟s methanolic extract was taken and then dissolved in DMSO. The concentration was adjusted to 320µg/ml by adding 3.9 ml DMSO and considering as the parent solution. A sequence of decreasing concentrated solutions was prepared by sequential dilution with std. DMSO. Then 50µl from each of these test solutions were withdrawn and added to premarked glass vials/test tubes containing 4.5ml of simulated seawater and 10 shrimp nauplii. So, the final concentrations were 320µg/ml, 160µg/ml, 80µg/ml, 40µg/ml, 20µg/ml, 10µg/ml, 5µg/ml, 2.5µg/ml, and 1.25µg/ml.
Preparation of control group
In cytotoxicity study, test methods should be validated by using control groups. This ensures that only the tested agents participated and effects of the other potential factors are abolished. Two types of control groups are normally used,
- Positive control
- Negative control
Preparation of the positive control group
In cytotoxicity study, positive control group gets much importance. It is not only accepted as a cytotoxic agent as the tested result is also evaluated with the result obtains from the positive control group. In this cytotoxicity study, vincristine sulphate was used as the positive control group. Precise quantity of the vincristine sulphate was dissolved in std. DMSO for getting an initial concentration of 20µg/mL. Serial dilutions are made using std. DMSO for getting the concentrations 10 µg/mL, 5µg/mL, 2.5µg/mL, 1.25µg/mL, 0.625µg/mL, 0.3125µg/mL, 0.15625µg/mL, 0.078125µg/mL, and 0.0390µg/mL. After that positive control groups were obtained by adding the positive control solutions to the premarked vials containing ten living brine shrimp nauplii in 5 mL simulated sea water.
Preparation of the negative control group
In the preparation of negative control group, 100µL of DMSO was added to each of three premarked glass vials/test tubes, which contain 5 mL of simulated sea water and 10 shrimp nauplii. The rate of mortality of brine shrimps in these vials/test tubes should not be very rapid. If it is, then the test will be considered as invalid as the nauplii may died due to some other reasons but not only for the cytotoxicity of the compounds.
Counting of nauplii
The vials/test tubes were inspected and the numbers of survivors were counted subsequent to 24 hours, by using a magnifying glass. The percent (%) of mortality was calculated for each dilution, individually. Linear regression was used by using a simple IBM-PC program to analyze statistically the concentration-mortality data. The median lethal concentration (LC50) value indicates the either effectiveness or the concentration-mortality relationship of plant product. Phytochemical and Biological Evaluation of Albizia richardiana benth. Fabaceae family 31 After a certain exposure period, the concentration of the chemical which produces death in half of the test subjects is represented by this value.
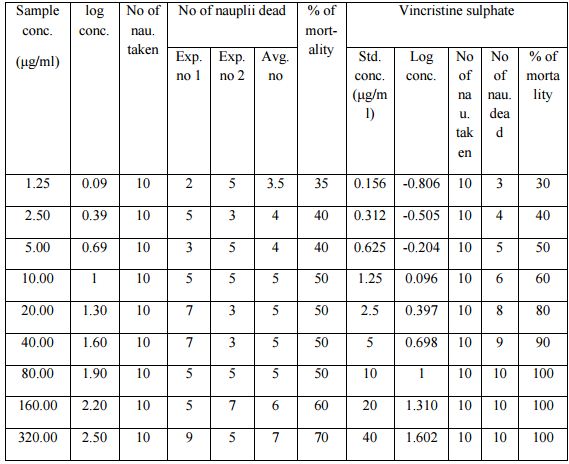
Results and discussion of Brine Shrimp Lethality Bioassay
The degree of lethality was directly proportional to the concentration of the extract, ranging from the lowest concentration (1.25 μg/ml) to the highest concentration (320μg/ml) (Table). The methanolic extract of the plant material had LC50 values of 22.03μg/ml while the LC50 of the reference anticancer drug, vincristine sulphate was 0.52μg/ml (Table). With increasing concentration of the sample the rate of mortality of the nauplii had increased.
Table:- Effect of methanolic extract of Albizia richardiana on brine shrimp lethality test in Artemia salina
Conclusion
The methanolic extract of bark of Albiziarichardiana (Fam. Fabaceae, subfam. Mimosoideae)wasinvestigated for the evaluation of phytochemical profile as well as biological profile of this plant.
The result of the present study shows that the plant contains some of the important bioactive constituents like more instance- carbohydrate, glucosides, glycosides, saponin and alkaloids. It was also observed from the investigation that the plant extract not responded positively in brine shrimp lethality bioassay with LC50value of about 22.03µg/ml. However, no statistically significant hypoglycemic activity of methanolic bark extract of Albizia richardiana, at both 200 mg/kg and 400 mg/kg body wt doseswas observed.
Thus, the present study has revealed potential bioactivity of Albiziarichardiana (Fam. Fabaceae, subfam. Mimosoideae), encouraging further extensive studies to find out their unexplored efficacy and to rationalize it as a traditional medicine.