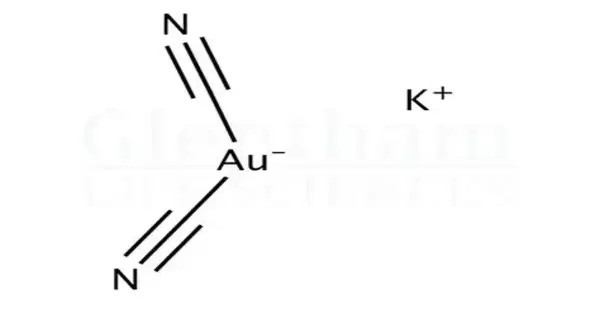
Potassium dicyanoaurate (or potassium gold cyanide) is an inorganic compound with formula K[Au(CN)2]. It is a coordination compound of gold. It is a colorless to white solid that is soluble in water and slightly soluble in alcohol. The salt itself is often not isolated, but solutions of the dicyanoaurate ion ([Au(CN)2]−) are generated on a large scale in the extraction of gold from its ores.
It consists of a potassium cation (K⁺) and the dicyanoaurate anion, [Au(CN)₂]⁻, where a gold(I) ion is linearly coordinated by two cyanide ligands. This compound is typically obtained as a white to colorless crystalline solid and is highly soluble in water. It plays a crucial role in gold extraction and refining processes, particularly in cyanidation, where gold from ores is converted into soluble dicyanoaurate complexes for recovery.
Properties
It appears as a crystalline solid, usually colorless to pale yellow. The compound is highly soluble in water, forming stable solutions. Structurally, the gold atom is linearly coordinated by two cyanide ligands. It is relatively stable in alkaline conditions but can decompose under acidic environments, releasing toxic hydrogen cyanide (HCN). The compound is toxic due to its cyanide content and requires careful handling.
- Chemical formula: KAu(CN)2
- Molar mass: 288.101 g/mol
- Appearance: white crystal
- Density: 3.45 g/cm3
- Boiling point: decomposes
- Solubility in water: 140 g/L
Production
In mining of gold from dilute sources, gold is selectively extracted by dissolution in aqueous solutions of cyanide, provided by dissolving sodium cyanide, potassium cyanide and/or calcium cyanide. The reaction for the dissolution of gold, the “Elsner Equation”, is:
4 Au + 8 KCN + O2 + 2 H2O → 4 K[Au(CN)2] + 4 KOH
In this process, oxygen is the oxidant.
It can also be produced by reaction of gold(I) salts with excess potassium cyanide.
AuCl + 2 KCN → K[Au(CN)2] + KCl
In structure, the gold is in the +1 oxidation state and exhibits a strong linear geometry due to the nature of cyanide ligands. The salt is stable under neutral or mildly basic conditions but decomposes in acidic environments, releasing toxic hydrogen cyanide (HCN) gas. Because of the cyanide content, potassium dicyanoaurate is highly toxic and must be handled with extreme care.
Occurrences
Potassium dicyanoaurate does not occur naturally; it is a synthetic compound primarily produced through the dissolution of metallic gold in potassium cyanide under oxidizing conditions. This process is the basis of the cyanidation method widely used in gold extraction and refining industries. Beyond metallurgy, it has applications in electroplating of gold, where it serves as a source of gold ions for depositing thin, uniform layers of gold on metal surfaces. Occasional uses include laboratory research in coordination chemistry and catalysis.
Uses
Dicyanoaurate is the soluble species that is the focus of gold cyanidation, the hydrometallurgical process for winning gold from dilute ores. In fact, sodium cyanide, not the potassium salt, is more widely used in commercial processes. Aside from its major use as an intermediate in the extraction of gold, potassium dicyanoaurate is often used in gold plating applications.
Beyond metallurgy, it has applications in electroplating, where thin, uniform layers of gold are deposited onto surfaces. It can also be used in certain analytical chemistry contexts to study coordination complexes. Its importance lies in being a practical, water-soluble form of gold that can be used in large-scale industrial and laboratory processes.