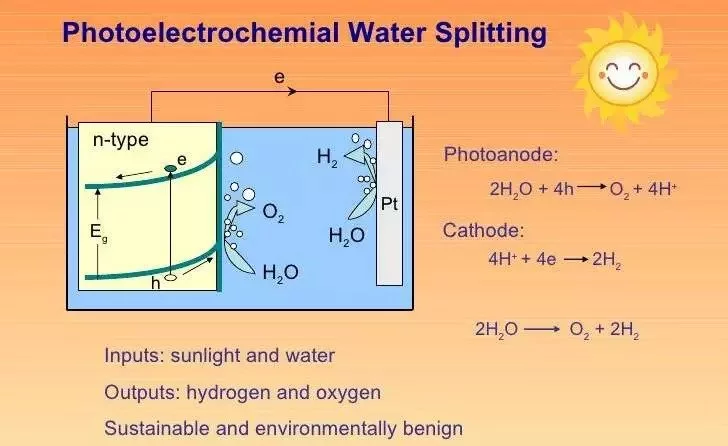
Photoelectrolysis of water, also known as photoelectrochemical water splitting, occurs in a photoelectrochemical cell when light is used as the energy source for the electrolysis of water, producing dihydrogen that can be used as a fuel. It is a process that uses light energy, typically from sunlight, to split water molecules (H2O) into hydrogen gas (H2) and oxygen gas (O2) via electrolysis. This process is one path toward a “hydrogen economy,” in which hydrogen fuel is produced efficiently and cheaply from natural sources without the use of fossil fuels. This is a promising technology for producing renewable hydrogen, which can be used as a clean and sustainable fuel source.
Steam reforming, on the other hand, almost always uses a fossil fuel to produce hydrogen. For its potential to yield a viable alternative to petroleum as a source of energy, photoelectrolysis is sometimes referred to colloquially as the hydrogen holy grail; such an energy source would allegedly come without the sociopolitically undesirable effects of extracting and using petroleum.
Photoelectrolysis on a nanoscale has been attempted by some researchers. Nanoscale photoelectrolysis of water may one day be more efficient than “traditional” photoelectrolysis. Semiconductors with bandgaps less than 1.7 eV are ostensibly required for efficient nanoscale photoelectrolysis using solar light.
Here’s how photoelectrolysis of water works:
- Photoactive Material: A photoactive material, also known as a photoelectrode or photocatalyst, is an important component of the photoelectrolysis process. Photons (light particles) from a light source, typically sunlight, are absorbed by this material and converted into electrons.
- Generation of Electron-Hole Pairs: Photons with sufficient energy excite electrons within the photoactive material, causing them to move to a higher energy state. This results in electron-hole pairs, in which an electron leaves its original position in the material (the hole).
- Electron Transport: The excited electrons are then transported through the photoactive material. This movement of electrons is critical for the subsequent electrolysis of water.
- Water Splitting: The photoactive material is immersed in water. The excited electrons, which have moved through the material, are used to reduce water at the cathode (negative electrode), where they combine with protons (H+) from the water to form hydrogen gas (H2). 2H+ + 2e- -> H2
- Oxygen Evolution: At the anode (positive electrode), the remaining holes from the electron-hole pairs in the photoactive material attract electrons from the water, leading to the oxidation of water and the release of oxygen gas (O2). 2H2O -> O2 + 4H+ + 4e-
The potential for renewable and clean hydrogen generation, scalability, and the ability to harness sunlight, a nearly limitless energy source, are all advantages of photoelectrolysis of water for hydrogen production. However, there are obstacles to overcome, such as increasing photoelectrode efficiency, lowering material costs, and ensuring long-term stability for practical applications. Researchers are working to improve this technology in order to make it more economically viable and efficient for large-scale hydrogen production. Hydrogenase-based devices have also been investigated.