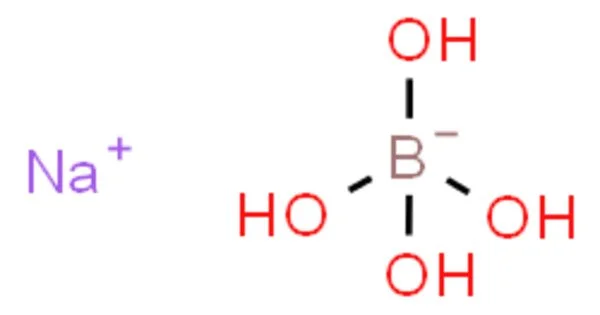
Sodium tetrahydroxyborate is a chemical compound with the formula NaB(OH)4. It is a salt (ionic compound) of with chemical formula NaH4BO4 or Na+[B(OH)4]−. It is one of several sodium borates. At room temperature it is a colorless crystalline solid. It is also known as sodium borate tetrahydrate or sodium borohydrate.
The element ratio corresponds to the oxide mixture Na2O·B2O3·4H2O, but the structure of the solid is quite different from that suggested by this formula.
Properties
Sodium tetrahydroxyborate is a white crystalline powder that is soluble in water and has a sweet taste. It is commonly used as a reducing agent in organic chemistry and as a source of boron in the production of boron carbide and other boron-containing compounds.
- Appearance: It is a white crystalline powder.
- Solubility: It is highly soluble in water and slightly soluble in alcohol.
- Stability: It is stable under normal conditions, but it can decompose upon heating, releasing hydrogen gas.
- Reducing agent: It is a strong reducing agent and is commonly used as a source of hydrogen in chemical reactions.
Applications
Sodium tetrahydroxyborate is used in various industries, including pharmaceuticals, agriculture, and as a reducing agent in the synthesis of organic compounds.
In addition, sodium tetrahydroxyborate has been investigated for its potential as a hydrogen storage material due to its ability to release hydrogen gas upon exposure to water. However, its use as a hydrogen storage material is still limited by practical challenges such as slow hydrogen release kinetics and issues with water management. Overall, sodium tetrahydroxyborate has a wide range of applications in various fields, including chemistry, materials science, and energy storage.
Safety
It is considered a hazardous substance and should be handled with care. It can be harmful if ingested or inhaled, and can cause skin irritation upon contact.
Storage
Sodium tetrahydroxyborate should be stored in a cool, dry place away from sources of heat and ignition. It should be kept in a tightly closed container to prevent moisture absorption.


![Report on Primary School Dropouts The Reasons Behind an Anthropological Investigation [part-4]](https://assignmentpoint.com/wp-content/uploads/2013/04/images-8-110x55.jpg)










