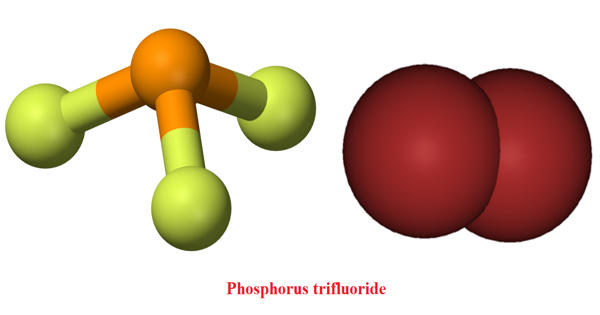
Phosphorus trifluoride (PF3), is a colorless and odorless gas. It appears as a colorless or slightly yellow fuming liquid with a pungent and irritating odor resembling that of hydrochloric acid. It is highly toxic and reacts slowly with water. Its main use is as a ligand in metal complexes. As a ligand, it parallels carbon monoxide in metal carbonyls, and indeed its toxicity is due to its binding with the iron in blood hemoglobin in a similar way to carbon monoxide. It is very toxic by inhalation, ingestion, and skin absorption.
This gas was prepared in 1884 by heating lead fluoride with copper phosphide in a brass tube. It is also produced by the action of fluorides of zinc, silver or lead on phosphorus trichloride or tribromide.
Physical properties
Phosphorus trifluoride has an F−P−F bond angle of approximately 96.3°. Gaseous PF3 has a standard enthalpy of formation of −945 kJ/mol (−226 kcal/mol). The phosphorus atom has a nuclear magnetic resonance chemical shift of 97 ppm (downfield of H3PO4). It is highly toxic and reacts slowly with water.
- Molecular weight: 87.97
- Form: liquid
- Appearance: gas
- Sensitivity: moisture
- Melting point: -151.5C
- Boiling point: -101.5C
Synthesis
Phosphorus is a poisonous yellowish-white chemical element. It glows slightly, and burns when air touches it. Phosphorus trifluoride hydrolyzes especially at high pH, but it is less hydrolytically sensitive than phosphorus trichloride. It does not attack glass except at high temperatures, and anhydrous potassium hydroxide may be used to dry it with little loss. With hot metals, phosphides and fluorides are formed. With Lewis bases such as ammonia addition products (adducts) are formed, and PF3 is oxidized by oxidizing agents such as bromine or potassium permanganate.
Ordinary phosphorus is a toxic flammable phosphorescent white solid; the red form is less reactive and nontoxic: used in matches, pesticides, and alloys.
Preparation
Phosphorus trifluoride is usually prepared from phosphorus trichloride via halogen exchange using various fluorides such as hydrogen fluoride, calcium fluoride, arsenic trifluoride, antimony trifluoride, or zinc fluoride:
2 PCl3 + 3 ZnF2 → 2 PF3 + 3 ZnCl2
Phosphorus trifluoride can also be made by the action of lead fluoride on phosphorus trichloride and by the decomposition of the pentafluoride by means of electric sparks.
Uses
It is used during electrodeposition of metal on rubber and for making pesticides, surfactants, gasoline additives, plasticizers, dyestuffs, textile finishing agents, germicides, medicinal products, and other chemicals.
Its main use is as a ligand in metal complexes. As a ligand it parallels carbon monoxide in metal carbonyls, and indeed its toxicity is due to the fact that it binds with the iron in blood haemoglobin in a similar way to carbon monoxide.
Information Source: