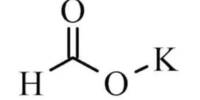
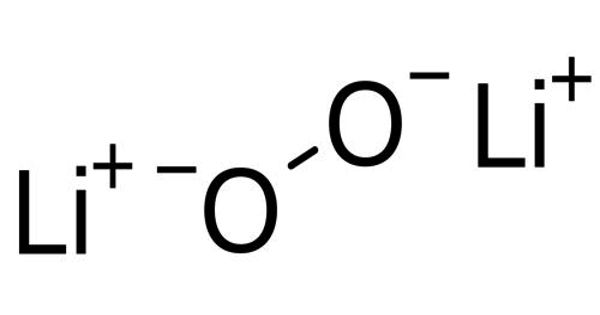Phosphate mineral is a group of naturally occurring inorganic salts of phosphoric acid, H3(PO4). These minerals contain the tetrahedrally coordinated phosphate (PO43−) anion along sometimes with arsenate (AsO43−) and vanadate (VO43−) substitutions. Chlorine (Cl−), fluorine (F−), and hydroxide (OH−) anions that also fit into the crystal structure. More than 200 species of phosphate minerals are recognized, and structurally they all have isolated (PO4) tetrahedral units.
The element phosphorus is very important for many facets of life. It is essential for the creation of DNA, cell membranes, and for bone and teeth formation in humans. It is ubiquitous although a minor component of most igneous and metamorphic rocks. The phosphate class of minerals is a large and diverse group, however, only a few species are relatively common. Phosphates can be grouped as:
(1) primary phosphates that have crystallized from a liquid;
(2) secondary phosphates formed by the alteration of primary phosphates; and
(3) fine-grained rock phosphates formed at low temperatures from phosphorus-bearing organic material, primarily underwater.
Applications
Phosphate rock has a high concentration of phosphate minerals, most commonly of the apatite group. It is the major resource mined to produce phosphate fertilizers for the agriculture sector. Phosphate mineral formation represents another phosphorus sink that is influenced by microbial activity; however, it encompasses processes other than cellular assimilation and short-term storage of phosphorus as biological molecules.
Phosphate is also used in animal feed supplements, food preservatives, anti-corrosion agents, cosmetics, fungicides, ceramics, water treatment, and metallurgy. The largest use of minerals mined for their phosphate content is the production of fertilizer. At first, bones, which contain the element phosphorus, were used as an agricultural fertilizer. Large numbers of phosphate minerals occur in the oxidized zones of base metal orebodies. Today, phosphate rock provides the phosphorus element of the nitrogen-phosphorus-potassium mix that fertilizer provides for plants. Solubility phenomena play the most important role in determining which phosphates crystallize in these low-temperature environments, where generally acidic groundwaters dominate.
Phosphate minerals are often used for control of rust and prevention of corrosion on ferrous materials applied with electrochemical conversion coatings. Phosphate is also used in animal feed supplements, food preservatives, anti-corrosion agents, cosmetics, fungicides, ceramics, water treatment, and metallurgy. The production of phosphate mineral fertilizers is one of the most profitable and financially stable among manufacturing industries.