Chemists created so-called heteroatom-substituted cage-like 3D molecules. The novel structures are formed by accurately placing a triatomic unit into the strained ring of a reaction partner. They may assist address important issues in drug design by providing more stable alternatives to typical, flat, aromatic rings.
As the name implies, ring-shaped “cage molecules” resemble cages, and their three-dimensional structure makes them substantially more stable than similar, flat molecules. As a result, they may be of interest to medication developers since they constitute a potential alternative to traditional molecular rings from the aromatic chemical family.
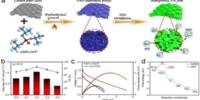
A research team at the University of Münster (Germany) led by chemist Prof Frank Glorius has developed a new method for producing so-called heteroatom-substituted 3D molecules and has published the results in the journal Nature Catalysis. The innovative structures are created by precisely inserting a triatomic unit into the strained (high-energy) ring of a reaction partner.
By using a light-sensitive catalyst, we were able to precisely insert nitrogen, oxygen and carbon atoms into this very reactive small bicyclic molecule which allowed us to synthesise a new type of 3D ring.
Prof Glorius
Aromatic rings are flat rings found in chemical compounds. They are among the most widely used compounds in medications and agrochemicals. However, these structures can be unstable under physiological settings, reducing the efficacy of pharmacological substances. To tackle this difficulty, scientists have been investigating sophisticated three-dimensional alternatives, such as cage-like rings that are stiffer and thus more stable.
While 3D equivalents for simple flat rings, such as benzene (a ring with six carbon atoms), are currently accessible, 3D copies of flat rings containing one or more other essential atoms, such as nitrogen, oxygen, or sulfur, have proven far more difficult to synthesize. These so-called “heteroaromatic” rings are especially prevalent in medicines.
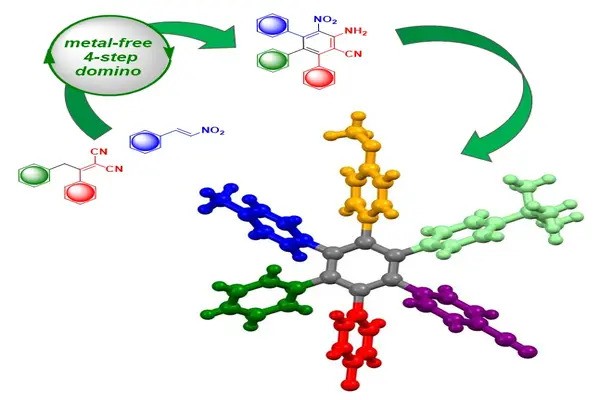
The breakthrough by the team from the University of Münster came by using bicyclobutane, a highly reactive molecule, and triggering the chemical reaction with light energy. “By using a light-sensitive catalyst, we were able to precisely insert nitrogen, oxygen and carbon atoms into this very reactive small bicyclic molecule which allowed us to synthesise a new type of 3D ring,” explains Prof Glorius.
Previous research focused mostly on adding carbon atoms into bicyclobutane. In contrast, introducing heteroatoms like nitrogen and/or oxygen results in new cage-like 3D rings. “These new rings could potentially serve as a substitute for flat heteroaromatic rings in drug molecules, opening up new possibilities for drug development,” says Chetan Chintawar, a doctor. The synthesised rings are stable, flexible, and easily changed, making them useful building blocks for many different cyclic compounds.
The researchers conducted both experimental and computational experiments to better understand the reaction’s mechanism. They propose that the reaction is launched by a light-induced electron transfer event from the excited catalyst to the reactants, which delivers the end products.