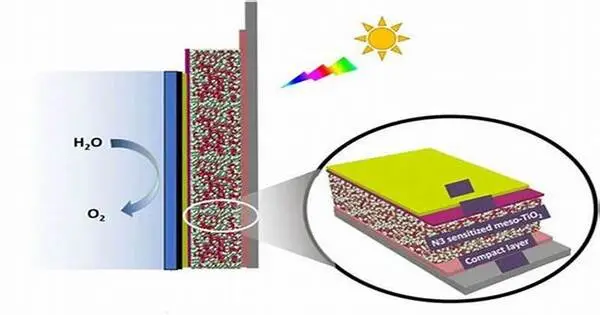
The field of solar hydrogen production was actively researching and developing new catalysts to improve the process’s efficiency and cost-effectiveness. Solar hydrogen production, also known as solar water splitting or photoelectrochemical water splitting, involves using sunlight to convert water into hydrogen and oxygen via a chemical reaction.
Researchers are working on a layered photocatalyst that can be used to produce hydrogen from water very efficiently. Finding sustainable and clean fuels is critical in today’s global energy and climate crisis. Hydrogen is a promising candidate that is gaining traction. However, today’s industrial hydrogen production still has a significant CO2 footprint, especially when processes like steam reforming or non-sustainable electrolysis are considered.
A team led by Prof. Dominik Eder of the Institute of Materials Chemistry (TU Wien) is thus focusing on the development of environmentally friendly processes for obtaining hydrogen, such as photocatalysis.
This process converts water molecules to hydrogen using only light and a catalyst. The sun’s abundant and clean energy can be stored in the chemical bonds of this so-called solarfuel via this process. The findings were recently published in the journal Advanced Energy Materials.
You can picture these layered structures as a Manner Schnitte, where the waffle is the inorganic part and the chocolate is the organic ligand holding them together. You just need to make the waffle part conductive.
Pablo Ayala
Novel photocatalysts
The catalyst is critical in the production of green hydrogen via photocatalysis. In contrast to industrial catalysts, a photocatalyst uses light energy to facilitate water splitting at room temperature and pressure. Metal-organic frameworks, or MOFs, are among the most promising candidates. They are made up of inorganic molecular building blocks held together by organic linker molecules. They combine to form highly porous 3D networks with an extremely large surface area and excellent charge separation properties.
Most MOFs, however, are only active when exposed to UV light, which is why the community modifies the organic compounds to allow them to absorb visible light. These changes, however, have a negative impact on electron mobility. Another limitation is the extraction of electrons from the material: “While MOFs are indeed great at separating charge carriers at the organic-inorganic interfaces, their efficient extraction for catalytic use remains a challenge,” Dominik Eder says.
Recently, MOFs with layered structures have caught a lot of attention for use in optoelectronic applications, as they exhibit greatly improved charge extraction properties. “You can picture these layered structures as a Manner Schnitte, where the waffle is the inorganic part and the chocolate is the organic ligand holding them together,” Pablo Ayala, lead author of the study, illustrates. “You just need to make the waffle part conductive.”
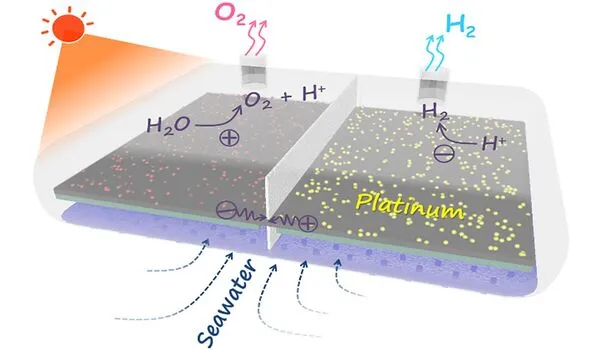
Challenges in water splitting
In contrast to 3D-MOFs, layered MOFs are typically non-porous, which reduces the catalytically active area to the particles’ external surface. “As a result, we needed to figure out how to make these particles as small as possible,” Eder explains. However, the introduction of structural defects is frequently accompanied by nanostructuring of materials. These can act as charge traps, slowing charge extraction.
“Nobody likes a Manner Schnitte with missing chocolate,” Ayala continues his analogy. “In the case of photocatalysis, we also need the best possible material that can be produced.”
As a result, Dominik Eder’s group devised a new synthesis route that allows even the smallest crystalline structures to be produced defect-free. This was accomplished with the help of local and international universities. The new, layered MOFs are titanium-based and have a cubic shape a few nanometers in size. The material has already set records in photocatalytic hydrogen production when exposed to visible light.
The team was able to unravel the underlying reaction mechanism with the help of computer simulations performed at Technion in Israel and demonstrated two things: First, that the layered nature of the MOF is indeed key to efficient charge separation and extraction. Second, that missing-ligand defects act as unwanted charge traps that need to be avoided as much as possible to enhance the material’s photocatalytic performance.