Chronic and neuropathic viral infections can cause neurodegenerative and neurobehavioral diseases, resulting in a loss of neurons and axons in the central nervous system that worsens with age. There is evidence of systemic viral infections occurring with some neurodegenerative conditions, such as Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, multiple sclerosis, autism spectrum disorders, and HIV-associated neurocognitive disorders, to date.
Some viral diseases may play a role in neurodegeneration. The findings were published in the scientific journal Nature Communications by DZNE researchers. Their conclusion is based on laboratory experiments in which they demonstrated that certain viral molecules promote the intercellular spread of protein aggregates, which are hallmarks of brain diseases such as Alzheimer’s. These findings could shed light on how acute or chronic viral infections can contribute to neurodegeneration.
Some viral diseases may play a role in neurodegeneration. Researchers discovered that certain viral molecules aided in the intercellular spread of protein aggregates, which are common in brain diseases such as Alzheimer’s. These findings could shed light on how acute or chronic viral infections can contribute to neurodegeneration.
Ina Vorberg
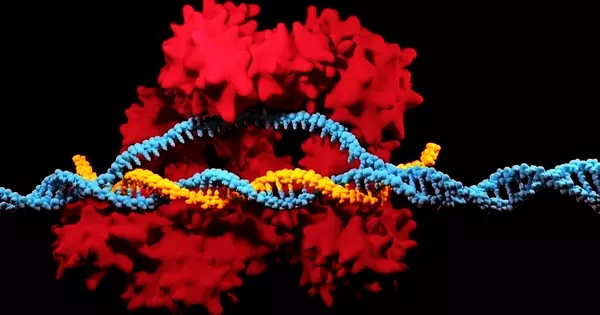
Misfolded protein aggregates, which occur in so-called prion diseases such as Creutzfeldt-Jakob disease, have the ability to pass from one cell to another, where they transfer their abnormal shape to proteins of the same kind. As a result, the disease progresses throughout the brain. A similar phenomenon is discussed for Alzheimer’s and Parkinson’s disease, which both exhibit misfolded protein assemblies. Direct cell-to-cell contact, the release of “naked” aggregates into extracellular space, or packaging in vesicles, which are tiny bubbles surrounded by a lipid envelope secreted for cell communication, could all be used for aggregate transmission.
“The precise mechanisms of transmission are unknown,” says Ina Vorberg, a research group leader at the DZNE’s Bonn site and a University of Bonn professor. “However, it is a foregone conclusion that aggregate exchange via direct cell contact and vesicles is dependent on ligand-receptor interactions. This is due to the fact that membranes must make contact and fuse in both scenarios. This is aided by the presence of ligands that bind to receptors on the cell surface, causing the two membranes to fuse.”
Experiments with Cell Cultures
Based on this assumption, Vorberg’s team conducted a series of studies with various cell cultures, with assistance from DZNE colleagues in Munich and Tübingen, as well as Belgian scientists. As a result, they looked into the intercellular transfer of either prions or tau protein aggregates, which occur in similar forms in prion diseases, Alzheimer’s disease, and other “tauopathies.”
The researchers induced cells to produce viral proteins that mediate target cell binding and membrane fusion, simulating what happens during viral infection. SARS-CoV-2 spike protein S, which is derived from the virus that causes COVID-19, and vesicular stomatitis virus glycoprotein VSV-G, which is found in a pathogen that infects cattle and other animals, were chosen as prime examples. Furthermore, cells expressed receptors for these viral proteins, specifically the LDL receptor family, which act as docking ports for VSV-G, and human ACE2, the spike protein receptor.
Ligands Facilitate Aggregate Spreading
“We were able to demonstrate that viral proteins are incorporated into both the cellular membrane and extracellular vesicles. Their presence increased the spread of protein aggregates between cells, either through direct cell contact or through extracellular vesicles. The viral ligands efficiently transferred aggregates into recipient cells, where they induced the formation of new aggregates. The ligands function as keys, unlocking the recipient cells and allowing the dangerous cargo to enter “Vorberg claims.
“Our cellular models, without a doubt, do not replicate the many aspects of the brain’s highly specialized cell types. The presence of viral ligands, on the other hand, clearly increased the spread of misfolded proteins to other cells, regardless of the tested cell type producing the pathologic aggregates. Overall, our findings suggest that viral ligand-receptor interactions can, in theory, influence pathologic protein transmission. This is a novel discovery.”
Potential Effects on Neurodegeneration
“Certain viruses have been found in the brains of patients suffering from neurodegenerative diseases. They are suspected of causing inflammation or having a toxic effect, thereby hastening neurodegeneration. However, viral proteins may behave differently: they may promote the intercellular spread of protein aggregates, which is already occurring in neurodegenerative diseases such as Alzheimer’s “Vorberg claims. “Of course, more research with neurotropic viruses is required. Clearly, the impact of viral infections on neurodegenerative diseases warrants further research.”