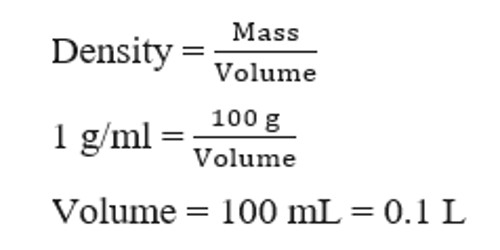Molar concentration, or molarity, or simply concentration, is a term in physical chemistry. It measures the concentration of a solution or mixture. The concentration of a solution is basically a measurement of solute (B) with respect to the solution(S) or solvent(A). It is the number of moles of solute per liter of solution, which can be calculated using the following equation: Molarity = mol solute/L of solution. It measures the quantity of a substance per unit volume. It is the most convenient method of expressing the concentration of a solute in a solution.
In chemistry, the molar concentration, Ci, is defined as the amount of a constituent, ni (usually measured in moles – hence the name) divided by the volume of the mixture, V: Ci = ni/V. It can be used to convert between the mass or moles of solute and the volume of the solution. One mole of solute in one liter of water gives a concentration of 1 M. For example, the acetic acid in the above example is completely dissolved in 1.25 L of water. Divide 0.1665 moles by 1.25 L to get the molar concentration, 0.1332 M. Specifically, it expresses the mole of a substance per liter of solution. The molar concentration follows the same rules that the other units in the International System of Units. The symbol is M or mol/L.
The molar concentration of a solute is defined as the number of moles of solute per liter of solution (not per liter of solvent). The volume, V in the definition, Ci = ni/V, refers to the volume of the solution, not the volume of the solvent. One liter of a solution usually contains either slightly more or slightly less than 1 liter of solvent because when a substance dissolves in a solvent it causes a volume of liquid to increase or decrease. The molar concentration of the solute is sometimes abbreviated by putting square brackets around the chemical formula of the solute.
The reciprocal quantity represents the dilution (volume) which can appear in Ostwald’s law of dilution.