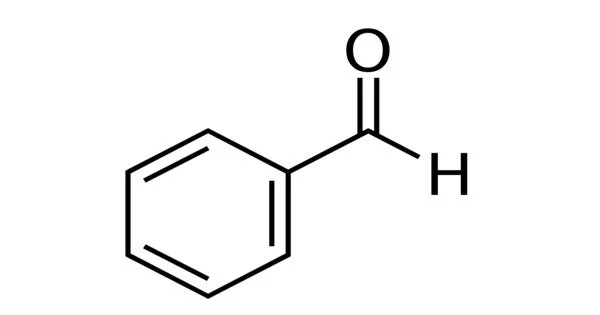
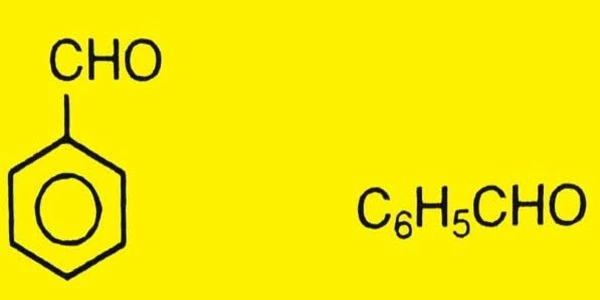
Benzaldehyde (C6H5CHO) is an organic chemical that is made when the hydrogen in benzene is replaced with an aldehyde. It is the simplest aromatic aldehyde and one of the most industrially valuable, consisting of a benzene ring with a formyl substituent. It’s an almond-scented aromatic aldehyde with a single formyl group. Benzaldehyde is a chemical that can be made from natural sources and is frequently used in the chemical industry to make aniline colors, fragrances, flavorings, and medicines.
Benzaldehyde is a white liquid with a distinct almond aroma. Benzaldehyde, the main component of bitter almond oil, can be derived from a variety of other natural sources. It is the most straightforward and widely used industrial aromatic aldehyde. Benzaldehyde is abundant in plants, particularly in the Rosaceae family. Amygdalin, bitter almond, cherry, laurel, and peach are examples of glycosides found in plant stem bark, leaves, or seeds.
The flavoring component in imitation almond extract, which is used to flavor cakes and other baked goods, is synthetic benzaldehyde. Bitter almond oil, patchouli oil, hyacinth oil, and cananga oil all contain it naturally. Amygdalin, which is a mixture of glycosides, can also be found in nutlets and nuts. Benzaldehyde has chemical characteristics that are similar to aliphatic aldehydes, but they are also distinct. It has a flash point of around 145°F, is denser than water, and is water insoluble. As a result, it sinks in water since vapors are heavier than air.
Properties
- Chemical formula: C7H6O
- Molar mass 106.124 g·mol−1
- Appearance colorless liquid
- strongly refractive
- Odor almond-like
- Density 1.044 g/mL, liquid
- Melting point −57.12 °C (−70.82 °F; 216.03 K)
- Boiling point 178.1 °C (352.6 °F; 451.2 K)
- Solubility in water 6.95 g/L (25 °C)
- Viscosity 1.321 cP (25 °C)
History
Martrès, a French pharmacist, was the first to extract benzaldehyde in 1803. His investigations were aimed at determining the nature of amygdalin, a deadly substance found in bitter almonds (Prunus dulcis). The fehling reagent cannot be reduced by benzaldehyde. The major products of using reducing fat to reduce benzaldehyde are benzene methanol, four substituted ortho-glycol, and two-phenyl ethylene glycol. By accepting the hydrogen atom in the presence of potassium cyanide, two molecules of benzaldehyde produce benzoin.
7000 tonnes of synthetic benzaldehyde and 100 tonnes of natural benzaldehyde were produced annually in 1999. The major routes are liquid phase chlorination and toluene oxidation. The meta-position product is the major product of the substitution reaction in the aromatic nucleus of benzaldehyde. When benzaldehyde is nitrated, for example, the major result is m-nitrobenzaldehyde. Other procedures, such as partial oxidation of benzyl alcohol, alkali hydrolysis of benzal chloride, and carbonylation of benzene, have been devised.
The simplest aromatic aldehyde and parent of the benzaldehyde class is benzaldehyde, which is an arenecarbaldehyde composed of benzene with a single formyl group. The retro-aldol reaction produces a significant amount of natural benzaldehyde from cinnamaldehyde obtained from cassia oil: the cinnamaldehyde is heated for 5 to 80 hours in an aqueous/alcoholic solution with a base (most commonly sodium carbonate or bicarbonate), followed by distillation of the formed benzaldehyde. Benzaldehyde undergoes autoxidation to perbenzoic acid in the absence of inhibitors, which interacts with a second molecule of benzaldehyde to give benzoic acid.
It has a role as a flavoring agent, a fragrance, an odorant receptor agonist, a plant metabolite, an EC 3.5.5.1 (nitrilase) inhibitor and an EC 3.1.1.3 (triacylglycerol lipase) inhibitor. It’s debatable whether benzaldehyde obtained this manner has a natural status. Other foods, such as masa flour, which is manufactured by processing corn flour with sodium hydroxide (lye), are exposed to undeniably more reactive circumstances. Condensation with aliphatic aldehydes provides additional aroma compounds or their unsaturated intermediates, and hydrogenation of benzaldehyde generates benzyl alcohol.
Chemical compound
Many foods contain benzaldehyde and other related compounds. The majority of benzaldehyde consumed comes from natural plant sources like almonds. Its bitter almond odor is employed in fragrance compositions. It’s the beginning point for a lot of araliphatic scent and flavor compounds. Benzaldehyde is absorbed by the skin and lungs, and it is distributed to all well-perfused organs. However, it does not accumulate in any one tissue type.
Cinnamic acid is produced by reacting benzaldehyde with anhydrous sodium acetate and acetic anhydride, while condensation of benzaldehyde to benzoin can be catalyzed by alcoholic potassium cyanide. A minor fraction of benzaldehyde in use is natural, while the great majority is synthetic. In essence, it is mostly used to provide taste to food. A little amount of benzaldehyde is used in the flavor and flavor of tobacco on a daily basis. Benzyl alcohol is still utilized to synthesis a number of different chemicals, from medications to plastic additives, despite its widespread use as a commercial food condiment and industrial solvent.
Applications
Benzaldehyde is mostly employed as a precursor to other chemical chemicals in industrial contexts, ranging from medications to plastic additives. Benzaldehyde and dimethylaniline are used to make the aniline color malachite green. It’s utilized in trace formulas for fragrances like lilac, white, violet, jasmine, acacia, sunflower, sweet plum, orange blossom, Tofu pudding, and so on; it’s also used in soap. Certain acridine dyes use benzaldehyde as a precursor. Benzaldehyde is transformed to cinnamaldehyde and styrene derivatives by aldol condensations.
If benzaldehyde is released to the environment, it will be broken down in air. It is thought that sunshine will break it down. From moist soil and water surfaces, it will migrate into the air. It should be able to travel freely through dirt. Gray and ductile cast iron (10 percent solution) and all amounts of lead are corroded by benzaldehyde. Pure benzaldehyde, on the other hand, is not corrosive to cast iron. Most common metals, such as stainless steels, aluminum, aluminum bronze, nickel and nickel-base alloys, bronze, naval brass, tantalum, titanium, and zirconium, are unaffected by benzaldehyde.
Benzaldehyde is also utilized as a flavoring in JUUL e-cigarette pods, especially in the “Cool Mint,” “Cool Cucumber,” and “Fruit Medley” flavors. In experimental animals, benzaldehyde is a mild skin irritant and a minor ocular irritant. In experimental animals exposed to modest quantities of benzaldehyde in the air, body weight was reduced and minor lung discomfort was detected. A technical technique for phenol produces some of the commercially available benzaldehyde. The oxidation of toluene to benzoic acid in air produces benzaldehyde as a by-product.
Information Sources: