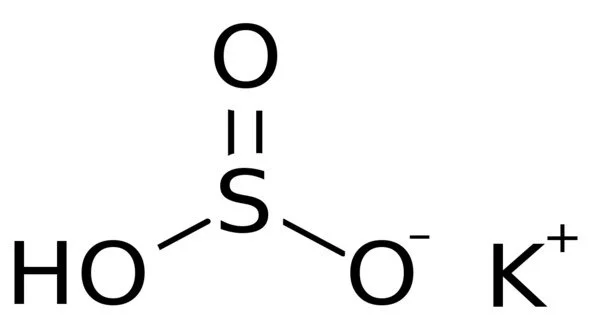
Potassium bisulfate is the potassium acid salt of sulfuric acid and is an inorganic compound with the chemical formula KHSO4. It is an acidic salt formed when sulfuric acid is partially neutralised with potassium hydroxide. It is a water-soluble white solid.
It is most commonly found as a white, crystalline powder or colourless crystals. It is extremely soluble in water. As the temperature rises, so does the solubility. It is an acidic salt that can release hydrogen ions (H+) in water, which causes it to be acidic. It is frequently used as a source of acidity in a variety of applications.
Properties
- Chemical formula: KHSO4
- Molar mass: 136.169 g/mol
- Appearance: colorless solid
- Odor: odorless
- Density: 2.245 g/cm3
- Melting point: 197 °C (387 °F; 470 K)
- Boiling point: 300 °C (572 °F; 573 K) (decomposes to form potassium pyrosulfate and water)
- Solubility in water: 36.6 g/100 mL (0 °C); 121.6 g/100 mL (100 °C)
- Solubility: soluble in acetone, ethanol.
Preparation
More than 1 million tons were produced in 1985 as the initial stage in the Mannheim process for producing potassium sulfate. The relevant conversion is the exothermic reaction of potassium chloride and sulfuric acid:
KCl + H2SO4 → HCl + KHSO4
Potassium bisulfate is a by-product in the production of nitric acid from potassium nitrate and sulfuric acid:
KNO3 + H2SO4 → KHSO4 + HNO3
Chemical properties
Thermal decomposition of potassium bisulfate forms potassium pyrosulfate:
2 KHSO4 → K2S2O7 + H2O
Above 600 °C potassium pyrosulfate converts to potassium sulfate and sulfur trioxide:
K2S2O7 → K2SO4 + SO3
Uses
Potassium bisulfate is commonly used to prepare potassium bitartrate for winemaking. Potassium bisulfate is also used as a disintegrating agent in analytical chemistry or as a precursor to prepare potassium persulfate, a powerful oxidizing agent.
- Electroplating: It is used in electroplating baths as a source of sulfate ions and to adjust the pH of the solution.
- Fertilizers: It can be used as a source of potassium and sulfur in fertilizers, providing essential nutrients for plant growth.
- Wine and beer production: It is used as a food additive in winemaking and brewing to inhibit microbial growth and preserve the quality of the final product.
- Water treatment: It is employed in water treatment processes to remove excess chlorine, adjust pH, and control alkalinity.
- Laboratory applications: It is used in various laboratory procedures and analytical techniques.
Occurrence
Mercallite, the mineralogical form of potassium bisulfate, occurs very rarely. Misenite is another more complex form of potassium bisulfate with the formula K8H6(SO4)7.
Safety considerations
Potassium bisulfate is generally considered safe when handled properly. However, it is acidic and can cause irritation to the skin, eyes, and respiratory system. It is important to follow appropriate safety precautions and guidelines when working with this compound.