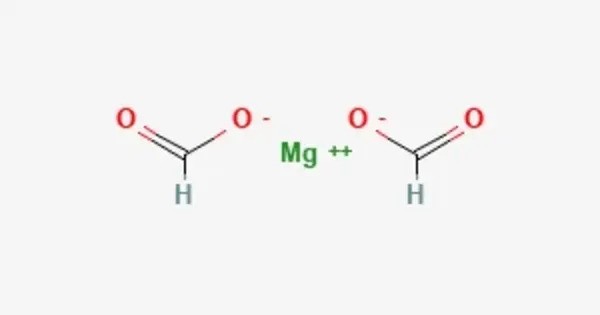
Magnesium formate is a magnesium salt of formic acid. It is an inorganic compound. It consists of a magnesium cation and formate anion. It can be prepared by reacting magnesium oxide with formic acid. The dihydrate is formed when crystallizing from the solution. The dihydrate dehydrates at 105 °C to form anhydrate, then decomposes at 500 °C to produce magnesium oxide. Magnesium formate can be used for organic syntheses.
It typically appears as a white, crystalline substance and is used in various applications, including as a supplement for magnesium, in the production of certain chemicals, and in agricultural products. Magnesium itself is an essential mineral for the human body, playing a key role in muscle and nerve function, bone health, and the regulation of blood sugar levels.
Properties
- Chemical formula: Mg(HCO2)2
- Solubility in water: 14 g/100g at 0 °C, 20.5 g/100g at 80 °C
- Physical state: It is typically a white, crystalline solid.
- Solubility: It is soluble in water, which is common for salts involving magnesium and small organic anions.
- Hygroscopic: It is hygroscopic, meaning it can absorb moisture from the air.
- Stability: It is generally stable under standard conditions, but it may decompose upon heating to release formaldehyde and magnesium oxide.
- Reaction with acids: It can react with strong acids to release formic acid.
Occurrences
- In nature: Magnesium formate does not occur naturally in large quantities, but magnesium compounds are abundant in nature, typically found in minerals like dolomite and magnesite.
- Industrial preparation: Magnesium formate can be synthesized in laboratories or industrial settings by reacting magnesium carbonate or magnesium oxide with formic acid.
- Uses: Magnesium formate is used in various applications, such as in the preparation of formate salts, in some chemical processes, and as a stabilizer in certain formulations.