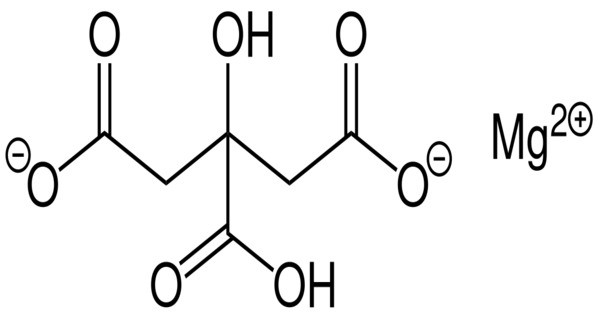
Magnesium citrates are metal-organic compounds formed from citrate and magnesium ions. They are salts. It is a magnesium supplement that is commonly used to help with magnesium deficiency, as well as to support various bodily functions like muscle and nerve function, energy production, and bone health. It is also used as a laxative to relieve constipation, as it has a mild purgative effect on the digestive system. One form is the 1:1 magnesium preparation in salt form with citric acid in a 1:1 ratio (1 magnesium atom per citrate molecule). It contains 11.33% magnesium by weight.
Magnesium citrate (sensu lato) is used medicinally as a saline laxative and to empty the bowel before major surgery or a colonoscopy. It is available without a prescription, both as a generic and under various brand names. It is also used in the pill form as a magnesium dietary supplement. As a food additive, magnesium citrate is used to regulate acidity and is known as E number E345.
Properties
- Chemical Formula: The chemical formula of magnesium citrate is typically C6H6MgO7C_6H_6MgO_7C6H6MgO7, though its exact form can vary depending on its hydration state.
- Appearance: It usually appears as a white, odorless, or nearly odorless crystalline powder.
- Solubility: Magnesium citrate is soluble in water, making it a good candidate for use in liquid supplements.
- pH: It is a weakly acidic compound due to the presence of citric acid.
- Magnesium Content: It provides a highly bioavailable form of magnesium, which is why it’s commonly used in supplements.
Occurrence
- Natural Occurrence: Magnesium citrate does not typically occur naturally in significant amounts. Instead, magnesium compounds, like magnesium carbonate or magnesium hydroxide, are found naturally in minerals such as magnesite and dolomite. Magnesium citrate is generally synthesized in the laboratory or industrial processes.
- Biological Context: Magnesium ions (Mg²⁺) naturally occur in biological systems, such as in plants, animals, and humans. Magnesium is crucial for many biological processes, including the functioning of enzymes, DNA replication, and muscle function. However, magnesium citrate itself is a synthesized product and doesn’t occur naturally in large quantities.
Use
Magnesium citrate is often used to treat magnesium deficiency and is commonly included in over-the-counter laxatives due to its ability to promote bowel movements. It’s also used in the food industry as a supplement.
Side effects
Magnesium citrate is generally not a harmful substance, but care should be taken by consulting a healthcare professional if any adverse health problems are suspected or experienced. Extreme magnesium overdose can result in serious complications such as slow heartbeat, low blood pressure, nausea, drowsiness, etc. If severe enough, an overdose can even result in coma or death.