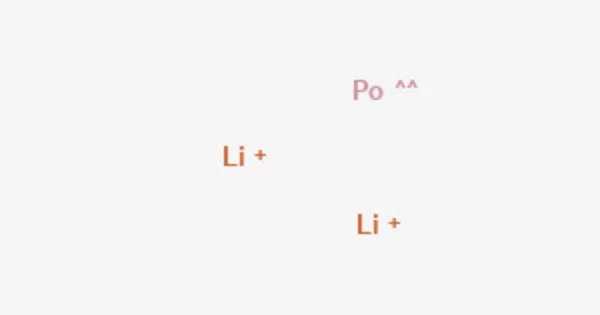
Lithium polonide is a chemical compound having the formula Li2Po. It is a polonide, which is a group of polonium compounds that are extremely chemically stable. It is classified as an intermetallic compound, which means it is formed when two or more metallic elements combine to produce a separate chemical combination. Because polonium is a scarce and extremely radioactive element, lithium polonide is uncommon.
LiPo is the chemical formula for lithium polonide. In this molecule, lithium (Li) gives one electron to form a positively charged ion (Li+), and polonium (Po) accepts one electron to generate a negatively charged ion (Po-). The ionic interaction between these ions leads in the production of lithium polonide.
Properties
- Radioactivity: It is extremely radioactive due to the presence of polonium, which is a highly radioactive element. The decay of polonium results in the emission of alpha particles.
- Color and Appearance: It is typically a gray or black solid, although its exact appearance can vary depending on the synthesis method and impurities present.
- Stability: It is not stable and tends to decompose over time, releasing radioactive decay products. It is highly reactive and should be handled with extreme caution.
- Melting Point: The melting point is not well-documented, but it is expected to be relatively low due to the reactivity of its components.
Production
Lithium polonide may be produced from a redox reaction between aqueous hydrogen polonide and lithium metal or from an acid-base reaction of H2Po with strong lithium-containing bases:
H2Po + 2 Li → Li2Po + H2
It may also be produced by heating lithium and polonium together at 300–400 °C. It is typically synthesized by reacting lithium with polonium. However, because of the scarcity and high radioactivity of polonium, its production is limited and strictly regulated.
Crystal structure
Like sodium polonide, lithium polonide has the antifluorite structure. Polonium is a radioactive element, and its most stable isotope, polonium-210 (Po-210), decays over time, emitting alpha particles. Due to its radioactivity, lithium polonide is also likely to be radioactive and hazardous to handle.
Uses
Outside of scientific research, lithium polonide has no practical applications. It is mostly used to explore the behavior of radioactive materials in nuclear physics and radiochemistry investigations.
Health and Safety
Lithium polonide poses substantial health risks due to its tremendous radioactivity and should be handled with extreme caution in a controlled laboratory environment. When working with this chemical, precautions such as lead shielding and remote handling are required.
Due to the uncommon and radioactive nature of polonium, research and knowledge on lithium polonide are restricted, and it is not a compound of significant commercial or industrial value. Because of the peculiar qualities of polonium and its radioactive decay characteristics, it is particularly of interest in nuclear physics and radiochemistry.