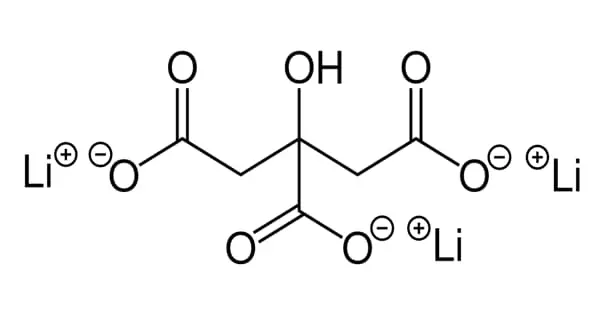
Lithium citrate (Li3C6H5O7) is a chemical compound composed of lithium and citrate. It is a lithium salt that is the anhydrous form of the trilithium salt of citric acid. It is a chemical compound composed of lithium and citrate that is used as a mood stabilizer in the psychiatric treatment of manic states and bipolar disorder. The tetrahydrate form is used to treat anxiety disorders, bipolar disorder, and depression. The active component of this salt, lithium, has lengthy pharmacology.
Lithium citrate is used to treat a variety of mental health issues that are thought to be caused by a chemical imbalance in the brain. It influences the levels and activity of certain chemicals in the brain. Lithium citrate is used to treat mania, mood disorders, and aggressive or self-harming behavior.
Properties
- Molecular Weight: 209.93
- Appearance: White crystalline powder
- Melting Point: 105 °C (dec.)
- Boiling Point: N/A
- Density: 1.12
- Solubility in H2O: N/A

Reactions
Lithium Citrate is a white, granular or crystalline powder that is slightly soluble in alcohol and soluble in water. It has the molecular formula C6H5Li3O7•4H2O. Its molecular weight is 282.0 and its chemical name is 1,2,3-Propanetri-carboxylic acid, 2-hydroxy-trilithium salt tetrahydrate.
Lithia water contains a number of lithium salts, including citrate. Lithia Coke, an early version of Coca-Cola sold in pharmacies’ soda fountains, was a combination of Coca-Cola syrup and lithia water. Because it contained lithium citrate, the soft drink 7Up was originally dubbed “Bib-Label Lithiated Lemon-Lime Soda” when it was introduced in 1929. The beverage was a patent medicine marketed as a hangover cure. In 1948, lithium citrate was removed from 7Up.
Uses
Lithium citrate is used to treat manic episodes of Bipolar Disorder. In the older DSM-II terminology, Bipolar Disorder, Manic (DSM-III) is equivalent to Manic Depressive Illness, Manic. It is also given to people suffering from certain types of depression when previous treatments have failed.
Warnings
When lithium is administered to a pregnant woman, it has the potential to harm the fetus. Lithium has been linked to adverse effects on nidations in rats, embryo viability in mice, and metabolism in vitro of rat testis and human spermatozoa, as well as teratogenicity in submammalian species and cleft palates in mice. Lithium-induced teratology has not been demonstrated in studies on rats, rabbits, or monkeys.