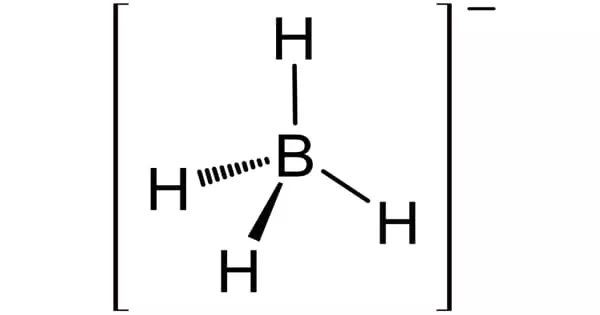
Lithium borohydride (LiBH4) is a borohydride that is used as a reducing agent for esters in organic synthesis. It is a powerful reducing agent. It can reduce esters to alcohols and primary amides to amines in methanol/diethyl ether mixtures. Although less common than sodium borohydride, lithium salt has some advantages, including being a stronger reducing agent and being highly soluble in ethers, while remaining safer to handle than lithium aluminum hydride.
Because of its higher chemoselectivity, this product is especially useful in some preparations. LiBH4 is frequently used in the reduction of aldehydes, ketones esters, lactones, and epoxides. Another use for this product is the synthesis of other borohydrides.
Properties
White orthorhombic crystals; density 0.67 g/cm3; decomposes in moist air; melts at 268°C; decomposes at 380°C; reacts with water; dissolves in ether, tetrahydrofuran, and diethylamine; solubility in ether, 25g/L at 25°C.
- Molecular Weight: 21.78
- Appearance: White to off-white powder, crystals, or crystalline powder
- Melting Point and Boiling Point: N/A
- Density: 0.666 g/cm3
- Solubility in H2O: N/A
- Form: Powder
- Color: White
- Specific Gravity: 0.66

Preparation
Lithium borohydride may be prepared by the metathesis reaction which occurs upon ball-milling the more commonly available sodium borohydride and lithium bromide:
NaBH4 + LiBr → NaBr + LiBH4
Alternatively, it may be synthesized by treating boron trifluoride with lithium hydride in diethyl ether:
BF3 + 4 LiH → LiBH4 + 3 LiF
Reactions
Lithium borohydride is a more powerful reductant than sodium borohydride. Lithium borohydride can reduce esters to alcohols and primary amides to amines in methanol/diethyl ether mixtures. Sodium borohydride, on the other hand, has no effect on these substrates. The polarization of the carbonyl substrate caused by complexation with the lithium cation is attributed to the increased reactivity.
Lithium borohydride reacts with water with liberation of hydrogen:
LiBH4 + 2H2O → LiBO2 + 4H2
Reaction with methanol yields lithium boromethoxide and hydrogen:
LiBH4 + 2CH3OH → LiB(OCH3)2 + 3H2
Reaction with hydrogen chloride yields diborane, lithium chloride, and hydrogen:
2LiBH4 + 2HCl → 2LiCl + B2H6 + 2H2
Reactions with oxidizing agents are violent.
Chemoselectivity
Because of its higher chemoselectivity compared to other popular reducing agents such as lithium aluminum hydride, the use of lithium borohydride is particularly advantageous in some preparations. Unlike lithium aluminum hydride, for example, lithium borohydride will reduce esters, nitriles, lactones, primary amides, and epoxides while sparing nitro groups, carbamic acids, alkyl halides, and secondary/tertiary amides.
Hydrogen generation
Lithium borohydride reacts with water to produce hydrogen. This reaction can be used for hydrogen generation.
Application
Lithium borohydride is an all-purpose reducing agent that is commonly used to reduce aldehydes, ketones esters, lactones, and epoxides. It catalyzes alkene hydroboration. It is also used to make other borohydrides, such as aluminum borohydride.
Its main uses are in organic synthesis to reduce carbonyl groups in aldehydes, ketones, and esters. It is also employed in the selective reduction of a carbonyl group in the presence of a nitrile group. Lithium aluminum hydride, a much stronger reducing agent, cannot achieve such selective reduction. The compound is also employed in the detection of free carboxyl groups in proteins and peptides.