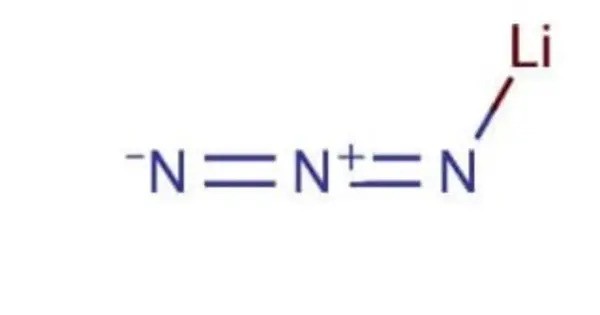
Lithium azide is the lithium salt of hydrazoic acid. It is an unstable and toxic compound that decomposes into lithium and nitrogen when heated. It is a highly reactive and potentially hazardous material, especially in the presence of heat or shock. It is commonly used in some chemical synthesis processes, including in the production of certain explosives, as it can decompose to release nitrogen gas and lithium compounds.
Because of its reactivity, lithium azide must be handled carefully, usually in controlled environments, to avoid dangerous situations. The compound has a high potential for detonation if exposed to the right conditions.
Preparation
It can be prepared by metathesis reaction between sodium azide and lithium nitrate or lithium sulfate solutions:
NaN3 + LiNO3 → LiN3 + NaNO3
2 NaN3 + Li2SO4 → 2 LiN3 + Na2SO4
It can also be prepared by reacting lithium sulfate with barium azide.
Ba(N3)2 + Li2SO4 → 2 LiN3 + BaSO4
Properties
It typically appears as a white solid or crystalline powder. It is slightly soluble in water but is more soluble in some organic solvents like acetone. It is known to decompose explosively when subjected to heat, shock, or friction. This makes it a material of concern in handling. It can react with acids and form hazardous gases, such as nitrogen.
- Chemical formula: LiN3
- Molar mass: 48.96 g·mol−1
- Melting point: 115 °C (239 °F; 388 K)
- Solubility in water: 36.12 g/100 g (10 °C), 66.41 g/100 g (16 °C)
- Solubility: 20.26 g/100 g (16 °C, ethanol)
Occurrences
- Natural Occurrence: Lithium azide is not found naturally in significant quantities. It is typically synthesized in laboratories or industrial settings.
- Synthetic Production: Lithium azide is commonly produced by reacting lithium metal with sodium azide (NaN₃), or lithium hydroxide with hydrazoic acid. It can also be synthesized by reacting lithium salts with sodium azide.
Uses
- Lithium azide has been explored in pyrotechnics and explosive formulations due to its instability and decomposition properties.
- It has been studied for use in propulsion systems (such as rocket motors) and in other high-energy applications.
Due to its sensitivity, lithium azide is handled under strict safety protocols. The compound is not commonly encountered in everyday products but is important in specialized fields like explosives, materials science, and pyrotechnics.