Fatty alcohols are usually high-molecular-weight, straight-chain primary alcohols, but can also range from as few as 4–6 carbons to as many as 22–26, derived from natural fats and oils. The precise chain length varies with the source. Some commercially important fatty alcohols are lauryl, stearyl, and oleyl alcohols. They are colorless oily liquids (for smaller carbon numbers) or waxy solids, although impure samples may appear yellow. They are similar to fatty acids in exhibiting amphiphilic properties and in the chemical and physical properties of the hydrophobic portion of the molecule, but with a nonionizable polar group.
Fatty alcohols usually have an even number of carbon atoms and a single alcohol group (–OH) attached to the terminal carbon. Some are unsaturated and some are branched. They are widely used in the industry. Conversion of fatty acids into fatty alcohols by catalytic hydrogenation without pre-esterification requires corrosion-resistant materials of construction and acid-resistant catalysts.
Production and occurrence
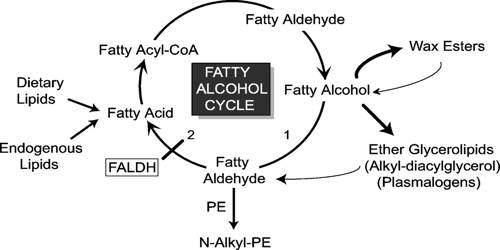
Most fatty alcohols in nature are found as waxes which are esters with fatty acids and fatty alcohols. They may be produced directly through the hydrogenation of fatty acids. They are produced by bacteria, plants, and animals for purposes of buoyancy, as a source of metabolic water and energy, biosonar lenses (marine mammals) and thermal insulation in the form of waxes (in plants and insects). However, the more common route of synthesis is through hydrogenation of methyl esters of fatty acids.
Fatty alcohols were unavailable until the early 1900s. They were originally obtained by the reduction of wax esters with sodium by the Bouveault–Blanc reduction process. In the 1930s catalytic hydrogenation was commercialized, which allowed the conversion of fatty acid esters, typically tallow, to the alcohols. In the 1940s and 1950s, petrochemicals became an important source of chemicals, and Karl Ziegler had discovered the polymerization of ethylene.
From natural sources
The traditional sources of fatty alcohol have largely been various vegetable oils and these remain a large-scale feedstock. They have been identified as promising oil-structuring agents. Animal fats (tallow) were of historic importance, particularly whale oil, however they are no longer used on a large scale. Tallows produce a fairly narrow range of alcohols, predominantly C16–C18, the chain lengths from plant sources are more variable (C6–C24) making them the preferred source. The alcohols are obtained from the triglycerides (fatty acid triesters), which form the bulk of the oil. Fatty alcohols occur as primary or secondary alcohols containing alkyl chains with unsaturated bonds or methyl branching groups. The process involves the transesterification of the triglycerides to give methyl esters which are then hydrogenated to give the fatty alcohols.
Applications
Fatty alcohols are mainly used in the production of detergents and surfactants. They are used extensively in cosmetic and personal care products in emulsion stabilization and also in providing structure for anhydrous sticks and suspensions. They are components also of cosmetics, foods, and as industrial solvents. Due to their amphipathic nature, fatty alcohols behave as nonionic surfactants. Fatty alcohols are found in many plants and animals, mostly as esters. They find use as co-emulsifiers, emollients and thickeners in cosmetics and food industry. About 50% of fatty alcohols used commercially are of natural origin, the remainder being synthetic.