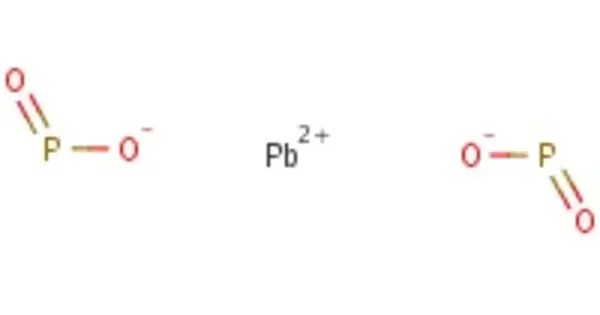
Lead heptaphosphide is the only binary phosphide currently known to be formed by lead and phosphorus. It has an unusual molecular structure where seven lead atoms are bonded to sixteen phosphorus atoms. This compound is a member of the family of phosphorus-rich lead compounds. The chemical formula is PbP7, which contains [P7]2− atom clusters. This compound is stable in the air.
Lead heptaphosphide is known for being highly unstable and is often synthesized under special conditions, such as in low-temperature environments. It is quite rare and does not have widespread applications in industry or research due to its instability. However, like many lead compounds, it can be toxic, and thus care must be taken in handling it.
Preparation
Lead heptaphosphide can be produced by the reaction of red phosphorus and lead:
7 P4 + 4 Pb → 4 PbP7
It decomposes again into the elements at 550 K (277 °C).
Properties
Lead heptaphosphide crystallises in the monoclinic crystal system, with space group P21/c, a=970.70(11), b=673.34(10), c=1243.89(18) pm and β=122.55(1)°. Each phosphorus atom in the phosphorus cluster is connected to the other six.
- Chemical formula: PbP7
- Molar mass: 424.03
- Appearance: black solid
- Color: It can appear dark in color (likely a metallic gray to black).
- Density: It is a relatively dense material due to the presence of lead, which has a high atomic mass.
- Melting Point: It has a relatively high melting point, though this can vary depending on the specific synthesis and impurities in the compound.
- Solubility: Like many lead-based compounds, it is not very soluble in water.
Chemical Properties
- Lead heptaphosphide is quite reactive, especially in the presence of acids or heat.
- It can decompose at high temperatures, and its reactivity is influenced by the oxidation states of lead and phosphorus.
- Lead tends to have oxidation states of +2 or +4 in its compounds, but the specific oxidation states in Pb₇P₁₃ can be more complex, depending on the electronic structure.
Occurrence
- Natural Occurrence: Lead heptaphosphide does not occur commonly in nature. It is typically synthesized in laboratory settings under controlled conditions. It may form in small amounts in areas with phosphorus and lead contamination, but it is not a naturally abundant mineral.
- Synthetic Occurrence: The compound is primarily produced in laboratories for study or for use in specific applications in materials science and electronics.
Applications
- While lead heptaphosphide has limited commercial use, compounds containing both lead and phosphorus can be important in the study of semiconductors and other electronic materials.
- Some research has explored the role of such compounds in new materials for batteries or other energy storage devices, though Pb₇P₁₃ itself is not widely used for these purposes.
Toxicity
As with many lead-based compounds, lead heptaphosphide is toxic and presents environmental hazards. Lead poisoning can occur if the material is ingested, inhaled, or if its dust is present in the air. Appropriate safety measures must be taken when handling it.