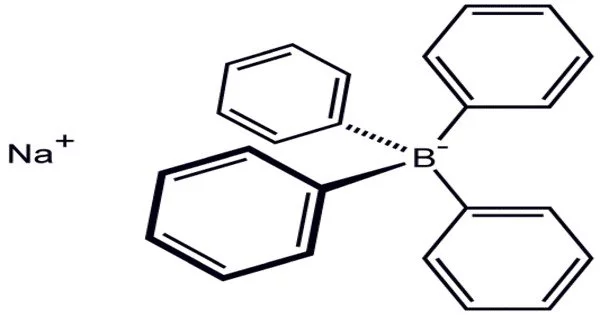
Sodium tetraphenylborate is a white crystalline powder with the chemical formula NaB(C6H5)4. It is a salt in which the anion is made up of four phenyl rings bonded to boron. It is a salt that is commonly used as a reagent in analytical chemistry to precipitate and isolate cations, particularly those of heavy metals such as lead, cadmium, and mercury.
Sodium tetraphenylborate is known for its high selectivity for certain cations, and its ability to form stable complexes with them. This white crystalline solid is used in the synthesis of other tetraphenylborate salts, which are frequently highly soluble in organic solvents. The compound is used as a precipitating agent for potassium, ammonium, rubidium, and cesium ions, as well as some organic nitrogen compounds, in inorganic and organometallic chemistry.
Properties
- Chemical formula: (C6H5)4BNa
- Molar mass: 342.216 g/mol
- Appearance: white solid
- Melting point: > 310 °C (590 °F; 583 K)
- Solubility in water: 47 g/100 mL
- Solubility: soluble in ethanol

Synthesis and structure
Sodium tetraphenylborate is synthesized by the reaction between sodium tetrafluoroborate and phenylmagnesium bromide:
NaBF4 + 4 PhMgBr → 2 MgBr2 + 2 MgF2 + NaBPh4 (where Ph = phenyl)
A related synthesis involves the use of phenylsodium in place of the Grignard reagent.
Unlike smaller counteranions, such as nitrate and the halides, tetraphenylborate confers lipophilicity to its salts. Many analogous tetraarylborates have been synthesized, containing both electron-rich and electron-deficient aryl groups.
The anhydrous salt adopts a polymeric structure in the solid state consisting of Na+-phenyl interactions. As such the salt could be classified as an organosodium compound.
Reactions
The precipitation reaction involving sodium tetraphenylborate occurs because the tetraphenylborate anion has a high affinity for cations, which allows for the formation of an insoluble complex that can be easily separated from the solution. This makes it a useful reagent in qualitative and quantitative analysis of metal ions in a wide range of samples, including environmental, biological, and industrial samples.
However, sodium tetraphenylborate can also interfere with the analysis of some other metal ions, such as zinc, copper, and iron, which limits its application in some cases. It is also important to handle it with care as it can be toxic if ingested or inhaled.
Uses
It is used as a reagent for the determination of cations, such as potassium, ammonium, and others in analytical chemistry, and also as a precipitating agent for heavy metals. It is important to handle Sodium Tetraphenylborate with caution, as it can be toxic if ingested or inhaled, and can cause skin and eye irritation upon contact.