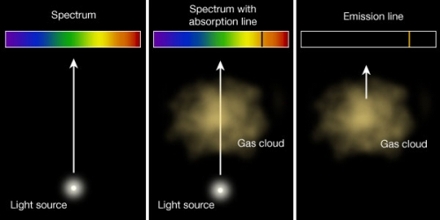
Kirchhoff’s Laws of Spectral Line focus on: Light of all wavelengths shines on an atom. Only light of an energy equal to the difference between “floors” will be absorbed and cause electrons to jump up in floors. The rest of the light passes on by to our detector. We see an absorption spectrum: light at all wavelengths minus those specific wavelengths. Kirchhoff’s Laws are: A hot solid, liquid or gas, under high pressure, gives off a continuous spectrum.
Kirchhoff’s Laws of Spectral Line