Isomerism is a process in which two or more compounds have the same chemical formula, but various structural formulas and distinct properties are available. Chemical compounds that have similar chemical formulas but vary in properties are called isomers and the arrangement of atoms in the molecule. Therefore, the compounds that exhibit isomerism are known as isomers. Greek “isos” plus “meros”, or “equal parts”, are the roots of the word ‘isomer’. Colloquially stated, isomers are chemical compounds that have the same components but are not the same anyway. In the year 1830, the Swedish chemist Jacob Berzelius coined this word.
Factors in isomerism also involve timing and electricity. Molecules are mobile entities that undergo all kinds of rotational motions that alter their shapes, and energy is required for those movements. Thus, on one timescale or set of energy conditions, some molecules may be the same, but on others, they may be different, or isomeric. Eventually, an isomer must be the minimum of energy; it must lie in a well of energy.
There are two major forms of isomerism that can be further divided into various subtypes. They are:
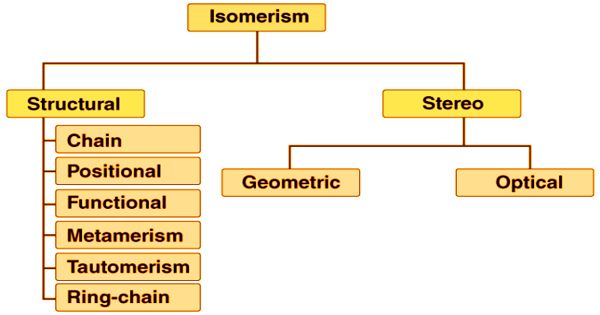
Structural Isomerism: Structural isomers (sometimes known as Constitutional isomers) are called isomers that vary in connectivity. They have the same parts, but those parts are connected differently to each other. The functional groups and the atoms are related in various ways in the molecules of these isomers. Isomers are molecules with the same molecular formula, but with distinct atomic arrangements in space. This excludes any distinct structures that are purely due to the molecule rotating as a whole or rotating on unique bonds. All of the above, for example, are the same molecule; they are not isomers. Another of the following forms is structural isomerism.
- Chain Isomerism: The probability of branching in carbon chains allows these isomers to arise. It is known as skeletal isomerism as well. The elements of these isomers present branched structures in different ways. For example, we can represent C5H12 as three compounds: CH3CH2CH2CH2CH3 – pentane
- Position Isomerism: Position isomer differs in the positions of the functional groups or substituent atoms. This isomerism usually includes the binding of the functional groups in the carbon chain to various carbon atoms. The basic carbon skeleton in place isomerism remains unchanged, however, on that skeleton important groups are shifted around. For example, we can represent C3H7OH in two arrangements: CH3CH2CH2OH – Propan-1-ol
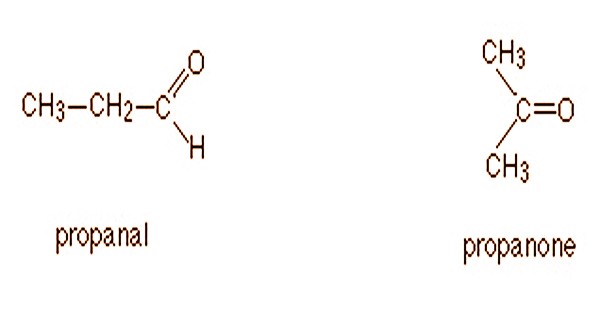
- Functional Isomerism: Isomers are functional isomers when there is an identical molecular formula for the two or more compounds that vary in the present functional group. It is also known as the isomerism of the functional group. As the name proposes, it alludes to the mixes that have similar compound recipe however unique practical gatherings connected to them. For example, A molecular formula C3H6O could be either propanal (an aldehyde) or propanone (a ketone).
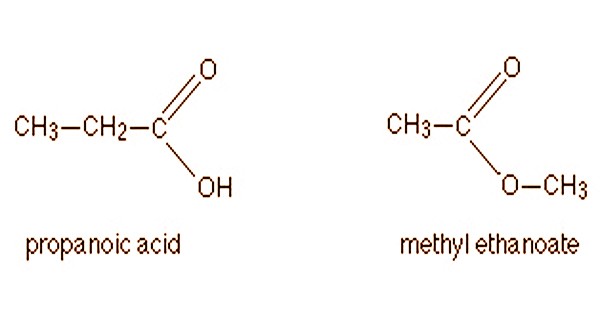
Another common example is illustrated by the molecular formula C3H6O2. Propanoic acid (a carboxylic acid) and methyl ethanoate (an ester) are among the few structural isomers here.
- Metamerism: Due to the presence of numerous alkyl chains on either side of the functional group, this kind of isomerism occurs. It is an uncommon form of isomerism and is normally limited to molecules that are surrounded by alkyl groups and contain a divalent atom (such as sulfur or oxygen). For example, we can represent C4H10O as ethoxyethane (C2H5OC2H5) and methoxypropane (CH3OC3H7)
- Tautomerism: A compound tautomer corresponds to the compound’s isomer, which varies only in the position of protons and electrons. Usually, in equilibrium and simple exchange, the tautomers of a compound exist together. It occurs through the transfer of an intramolecular proton.
- Ring-Chain Isomerism: One of the isomers has an open-chain structure in the ring-chain isomerism, while the other has a ring structure. Generally, they contain a different number of bonds with pi. A great example of this type of isomerism can be observed in C3H6.
Stereoisomerism: Stereoisomers, commonly defined, are isomers that have the same structure (i.e. the same parts) but vary in the orientation of those parts in space. Two forms of stereoisomers are present: enantiomers and diastereomers. Stereoisomers are also referred to as compounds that display stereoisomerism. However, timescale and energy are essential, as mentioned above. It is helpful to first consider a special type of stereoisomer, the conformational isomer, in order to appreciate these considerations. We may further categorize this phenomenon into two subtypes.
- Geometric Isomerism: It is shown by molecules in which, due to the presence of a ring structure or a double bond, their spatial positions are locked to each other. It is commonly referred to as cis-trans isomerism. In three-dimensional space, such isomers have different spatial arrangements of atoms.
- Optical Isomerism: Related bonds but different spatial configurations of atoms forming non-superimposable mirror images are compounds that show optical isomerism. Such optical isomers are known as enantiomers as well. Enantiomers vary in their optical activities from each other.
Information Sources: