Nuclear energy is essential for creating cleaner energy, but the radioactive contamination it produces calls for clever mitigation measures. Nuclear power plants produce the radioisotope cesium (Cs+), which must be immobilized and removed from the environment using high adsorption techniques.
Despite being top options for cleanup, phosphate-based adsorbents have a restricted capacity due to ineffective ion exchange. The observed adsorption capacity of phosphate adsorbents do not match their high theoretical adsorption.
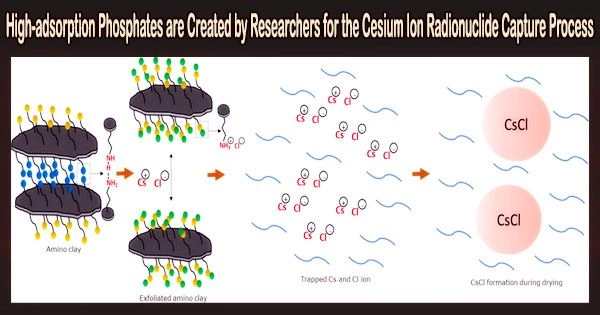
Researchers from Pusan National University working under the direction of Professor Kuk Cho from the Department of Environmental Engineering have created dittmarite-type phosphates with a layered structure that are perfect for simple ion exchange in order to remove dangerous Cs+ from radioactive wastewater.
Due to exchangeable ions and dissolution-precipitation, the team discovered that their magnesium phosphates exhibited record-high adsorption capabilities for Cs+, surpassing those of conventional adsorbents.
Cs+ is a popular radionuclide generated from nuclear power plants, and the volume of its waste must be minimized for disposal. To minimize the volume, the adsorbent with higher adsorption capacity is advantageous.
Professor Kuk Cho
Prof. Cho says, “The presence of exchangeable ions and dissolution-precipitation enabled record-high adsorption capacities for Cs+ that are higher than those of standard adsorbents.”
The study was published in the Journal of Hazardous Materials. Using a one-pot hydrothermal method, the team synthesized KMgPO4⋅H2O (KMP) and NH4MgPO4⋅H2O (NMP), both of which are dittmarite-type compounds, having a high theoretical adsorption capacity of 754 mg g- 1 and 856 mg g- 1 for Cs+, respectively.
The exceptional adsorption capacities of the synthesized KMP and NMP, which were 630 mg g-1 and 711 mg g-1, respectively, were 84% of their predicted adsorption capacities. The adsorption capacity values obtained through experimental measurement are the greatest for Cs+ among all known adsorbents.
The scientists then described and examined the phosphates’ chemical and physical characteristics. They demonstrated that these phosphates are not ideal for usage in water with high divalent ion concentrations based on the Cs+ adsorption performance of KMP and NMP.
However, they can still be used in Cs+ readsorption processes, following desorption processes, to concentrate Cs+ and reduce waste volume.
Prof. Cho says, “Cs+ is a popular radionuclide generated from nuclear power plants, and the volume of its waste must be minimized for disposal. To minimize the volume, the adsorbent with higher adsorption capacity is advantageous.”
According to the study, the novel phosphates effectively adsorb Cs+, offering a low-cost means of getting rid of radioactive waste. This is especially critical in a world where there are predicted to be more nuclear power plants, making effective storage with the right adsorbents essential for sustainability.
In conclusion, the synthesized phosphates are interesting candidates to address the problem of disposing of radioactive waste due to their high adsorption capabilities and stability.