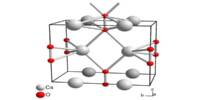
Graphitic carbon nitride (g-C3N4) is a polymeric material made up of C, N, and some impurity H that is linked together using tris-triazine-based patterns. It is a family of carbon nitride compounds with a general formula similar to C3N4 (albeit with non-zero amounts of hydrogen) and two major substructures based on heptazine and poly(triazine imide) units that exhibit varying degrees of condensation, properties, and reactivities depending on reaction conditions. It is a relatively new type of carbon-based material that has only recently been synthesized.
Properties
Carbon nitrides exhibit unexpected catalytic activity for a variety of reactions due to their unique semiconductor properties, including benzene activation, trimerization reactions, and carbon dioxide activation (artificial photosynthesis).
- Molecular Weight: Varies with composition
- Appearance: Off-white to yellowish-brown powder
- Melting Point: N/A
- Boiling Point: N/A
- Density: 2.336 g/cm3
- Average Particle Size: > 30 microns
- Specific Surface Area: >35 m2/g
- Solubility in H2O: N/A
Preparation
Polymerization of cyanamide, dicyandiamide, or melamine can yield graphitic carbon nitride. Melon, the first polymeric C3N4 structure formed, is a highly ordered polymer with pendant amino groups. The further reaction results in more condensed and less defective C3N4 species, with tri-s-triazine (C6N7) units as basic building blocks.
At room temperature, graphitic carbon nitride can also be prepared by electrodeposition on Si(100) substrate from a saturated acetone solution of cyanuric trichloride and melamine. Well-crystallized graphitic carbon nitride nanocrystallites can also be produced by a benzene-thermal reaction of C3N3Cl3 and NaNH2 at 180–220 °C for 8–12 h.
A new method of producing graphitic carbon nitrides by heating a mixture of melamine and uric acid in the presence of alumina at 400-600 °C has recently been reported. The deposition of graphitic carbon nitrides layers on the exposed surface was aided by the presence of alumina. This method is comparable to in situ chemical vapor deposition (CVD).
Characterization
X-ray photoelectron spectroscopy (XPS) measurements, photoluminescence spectra, and Fourier transform infrared spectroscopy (FTIR) spectrum (peaks at 800 cm1, 1310 cm-1, and 1610 cm-1) can be used to characterize crystalline g-C3N4 by identifying the triazine ring present in the products.
It is a semiconductor polymeric photocatalyst that has gained popularity in the field of visible-light-induced hydrogen evolution reaction (HER) due to its simple synthesis procedure, low bandgap energy, light absorption in the visible spectrum, easy functionalization, appealing electronic band structure, and high physicochemical stability.
Uses
Nicanite is the brand name for a commercial graphitic carbon nitride. It can be used in tribological coatings, biocompatible medical coatings, chemically inert coatings, insulators, and energy-storage solutions in its micron-sized graphitic form. One of the best hydrogen storage materials is graphitic carbon nitride. It can also serve as a platform for catalytic nanoparticles.