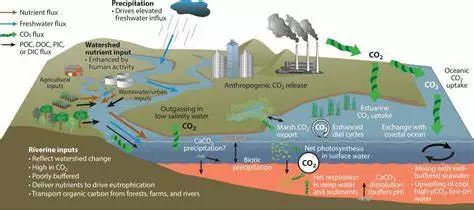
Estuarine acidification occurs when the pH equilibrium of water in coastal marine habitats, particularly estuaries, deteriorates. It refers to the process through which the pH levels of estuaries, which are partially enclosed coastal bodies of water where freshwater from rivers and streams meets and mixes with seawater, fall. Water, which is commonly called pH neutral, is normally completely balanced between alkalinity and acidity.
While ocean acidification is caused by the absorption of carbon dioxide (CO2) from the atmosphere, pH change in estuaries is more complicated than in the open ocean due to direct impacts from land run-off, human impact, and coastal current dynamics. Human actions, particularly the release of carbon dioxide (CO2) into the atmosphere and subsequent absorption into the ocean, are mostly responsible for this occurrence.
Carbonic acid (H2CO3) is formed when carbon dioxide (CO2) reacts with water (H2O) in the ocean due to wave and wind movement. This chemical link is jumbled up by wave motion, causing it to break further, finally becoming carbonate (CO3), which is basic and helps build shells for ocean organisms, and two hydron molecules. Because hydron ions readily connect with any Lewis Structure to produce an acidic bond, this provides the possibility of an acidic threat. This is called an oxidation-reduction reaction.
The basic chemical equation is as follows:
CO2 + H2O ⇌ H2CO3 ⇌ HCO3 + H+ ⇌ CO3 + 2 H+
However, when same absorption pattern is moved to an estuary, acidity increases simply due to relative volume. Ocean water absorbs 30-40% of all CO2 produced into the atmosphere, yet due to its enormous volume, it remains relatively resilient. Estuaries, due to their limited volume, protection from wave motion, and vulnerability to human impact when in an urban area, do not readily sustain water mixing, preventing basic breakdown.
Effects on Marine Life
Estuarine acidity, like ocean acidification, has the potential to harm aquatic species. Many marine animals rely on calcium carbonate to create their shells and skeletons, including shellfish, corals, and certain forms of plankton. The availability of carbonate ions, a crucial building ingredient of calcium carbonate, diminishes when the pH of estuarine waters lowers. This can make it more difficult for these species to create and maintain their protective structures, potentially threatening their survival as well as the ecosystem as a whole.
Ecosystem Impact
Estuaries are crucial ecosystems that support a diverse range of species, including those significant for commercial and recreational fishing. Changes in estuarine pH levels can disrupt food chains and alter the character of these ecosystems, potentially having economic and ecological ramifications.
When this is mixed with CO2 from human impact, such as car emissions or fertilizers, oxidation happens more rapidly due to an excess of hydron ions and extra cation, increasing the rate and duration of acidification. As the acidity of estuary water levels fluctuates, the reproduction levels of some species that use estuaries as spawning nurseries have decreased.