Diimide, also known as diazene or diimine, is a chemical compound with the formula (NH)2. It is found in two geometric isomers, E (trans) and Z. (cis). For organic diimide derivatives, the term diazene is more commonly used. As an example of an organic diazene, consider azobenzene.
Reductions with diimide are chemical reactions in which unsaturated organic compounds are converted to reduced alkane products. Diimide (N2H2) is oxidized to dinitrogen during the process. Many stereochemical studies have shown that diimide approaches from the less sterically hindered side of the double bond, implying that steric approach control dominates the stereoselectivity of the diimide reduction reaction.
Synthesis
A traditional route to diimide involves the oxidation of hydrazine with hydrogen peroxide or air. Alternatively, the decarboxylation of azodicarboxylic acid affords diimide:
(NCOOH)2 → (NH)2 + 2 CO2
Nowadays, diimide is generated by the thermal decomposition of 2,4,6‐triisopropylbenzenesulfonylhydrazide.
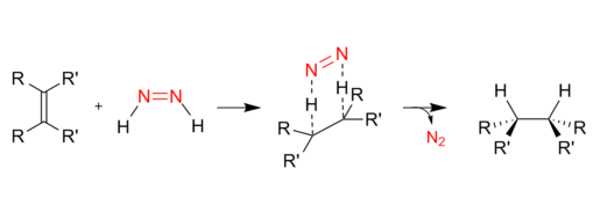
Diimide is generated and used in-situ due to its instability. The result is a mixture of the cis (Z-) and trans (E-) isomers. Both isomers are unstable, and they interconvert slowly. The trans isomer is more stable, but because the cis isomer reacts with unsaturated substrates, the equilibrium shifts towards the cis isomer due to Le Chatelier’s principle. Some procedures require the addition of carboxylic acids to catalyze cis-trans isomerization. Diimide is easily decomposable. Even at low temperatures, the more stable trans isomer undergoes rapid disproportionation reactions, primarily producing hydrazine and nitrogen gas:
2 HN=NH → H2N–NH2 + N2
Because of this competing decomposition reaction, reductions with diimide typically require a large excess of the precursor reagent.
Applications to organic synthesis
Diimide can be used as a reagent in organic synthesis on occasion. It hydrogenates alkenes and alkynes by delivering hydrogen selectively from one face of the substrate, resulting in the same stereoselectivity as metal-catalyzed syn addition of H2. Nitrogen gas is the only byproduct produced. Although the method is time-consuming, the use of diimide eliminates the need for high pressures, hydrogen gas, and metal catalysts, which can be costly.
Selectivity
Diimide is advantageous because it selectively reduces alkenes and alkynes while remaining unreactive to many functional groups that would interfere with normal catalytic hydrogenation. Thus, diimide tolerates peroxides, alkyl halides, and thiols, but these same groups are typically degraded by metal catalysts. The reagent preferentially reduces alkynes and unhindered or strained alkenes to alkenes and alkanes.