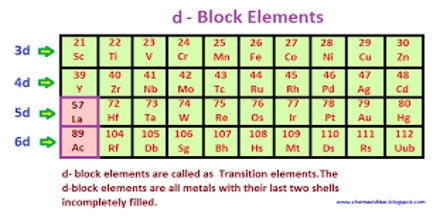
The d block consists of three horizontal series in periods 4, 5 and 6. Ten elements in each series. Basic purpose of this lecture is to present on d Block Elements; chemistry is “different” from other elements. Here briefly present on why special electronic configurations are important and differences within a group in the d block are less sharp than in s & p block and describe why similarities across a period are greater. Finally present on Electronic Configuration and transition metal.
d Block Elements