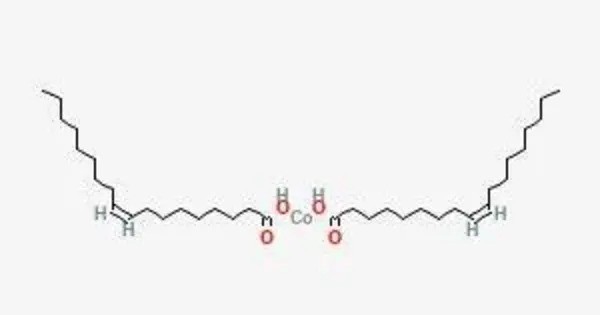
Cobalt oleate is an organometallic compound with the formula Co(C18H33O2)2. It is a coordination compound formed from cobalt and oleic acid. It is a chemical compound formed by the reaction of cobalt with oleic acid. When cobalt oleate is added to non-polar solvents, the viscosity rapidly increases, and then continues increasing over time. This unusual viscosity effect is caused by the formation of a weak coordination complex with the solvent molecules.
It’s often used as a drying agent in paints and coatings, helping to speed up the drying process of oils. In some applications, it can also be used as a catalyst or in the production of certain types of rubber and plastics.
Preparation
Cobalt oleate can be synthesized by heating a solution of sodium oleate and cobalt(II) chloride to 70 °C.
2NaC18H33O2 + CoCl2 ⟶ 2NaCl + Co(C18H33O2)2
Properties
Cobalt oleate is generally insoluble in water but can dissolve in organic solvents like ethanol or benzene. It usually presents as a dark blue or violet solid. It is relatively stable under normal conditions but may decompose upon exposure to extreme temperatures.
- Chemical formula: C36H66CoO4
- Molar mass: 621.853 g·mol−1
- Appearance: Purple powder
- Solubility: Soluble in benzene, carbon tetrachloride, pyridine, chloroform, quinoline
Occurrences
- Natural Sources: While cobalt oleate itself is not commonly found in nature, cobalt ions are often present in various minerals. Cobalt can react with oleic acid under specific conditions to form cobalt oleate.
- Synthetic Production: Cobalt oleate is primarily produced through the reaction of cobalt salts (like cobalt(II) chloride) with oleic acid. This can occur in laboratory settings or industrial applications.
Applications
It’s used in various fields, including as a drying agent in paints and inks, in ceramics, and in some catalytic processes.