Cobalt ferrite is a semi-hard ferrite with the chemical formula of CoFe2O4 (CoO·Fe2O3). It is a type of magnetic material composed of cobalt and iron oxide (CoFe₂O₄). The substance can be considered as between soft and hard magnetic material and is usually classified as a semi-hard material.
It has good chemical stability and resistance to corrosion, which is beneficial for various industrial applications. It is used in high-frequency applications like transformers and inductors, as well as in microwave devices, magnetic sensors, and magnetic recording media.
Properties
Cobalt ferrite is a hard magnetic material with high coercivity and saturation magnetization, making it suitable for applications in permanent magnets and magnetic recording media. It exhibits semiconducting behavior, which can vary with temperature and composition, influencing its use in electronic applications. It is chemically stable and resistant to oxidation, contributing to its durability in various environments.
- Chemical formula: CoFe2O4
- Molar mass: 234.619 g·mol−1
Occurrences
- Natural Occurrences: Cobalt ferrite can be found in some natural mineral deposits, typically in association with other iron and cobalt minerals.
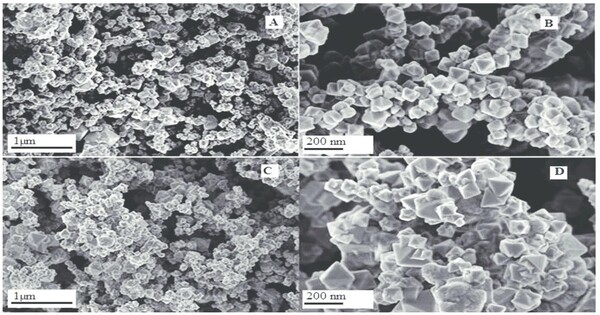
- Synthetic Production: It is often produced synthetically through various methods, including solid-state reaction, sol-gel process, and hydrothermal synthesis, allowing for control over particle size and morphology.
Applications
It is mainly used for its magnetostrictive applications like sensors and actuators thanks to its high saturation magnetostriction (~200 ppm). CoFe2O4 has also the benefits to be rare-earth free, which makes it a good substitute for Terfenol-D. Due to its magnetic properties, cobalt ferrite is widely used in electronics (like inductors and transformers), magnetic recording, and as a catalyst in chemical processes.