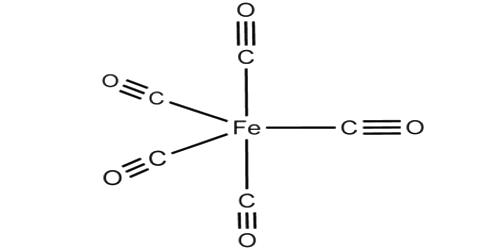
Carbonyl metallurgy is used to manufacture products of iron, nickel, steel, and other metals. Coatings are produced by vapor plating using metal carbonyl vapors. It is any complex of a transition metal combined with carbon monoxide; they are typically volatile solids or liquids, soluble on common organic solvents but insoluble in water; they are highly toxic. These are metal-ligand complexes where carbon monoxide is bonded to individual atoms of metals. Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. They occur as neutral complexes, as positively charged metal carbonyl cations or as negatively charged metal carbonylates. Some common metal carbonyls include- tetracarbonylnickel Ni(CO)4, pentacarbonyl iron Fe(CO)5, and octa-carbonyl dicobalt Co2(CO)8.
Iron carbonyl as stable as iron pentacarbonyl, where five carbon monoxide molecules are pendently bonded to the iron atom, while nickel carbonyl is stable as nickel tetracarbonyl, which has four carbon monoxide molecules pendently bonded to the nickel atom. Practical application is further advanced than a fundamental understanding of the processes. Both can be formed by the exposure of the powdered metal to carbon monoxide gas at temperatures of around 75 degrees Celsius. Both the metal carbonyls decompose near 175 °C, resulting in a vapor plated metallic coating. In general, the metal carbonyls are produced by the direct action of carbon monoxide on the finely divided metal. The thickness of the vapor plated deposit can be increased to the desired thicknesses by controlling the amount of metal carbonyl used and the duration of the plating process. They are used in the preparation of metals of exceptionally high purity and as catalysts in organic syntheses.

Carbonyl metallurgy is useful as a low-temperature metal coating technique that may find many applications in the future. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalyzes, such as hydroformylation and Reppe chemistry. The electronic structures of most metal carbonyls containing one metal atom per molecule (mononuclear carbonyls) bear striking resemblances to those of the noble-gas elements.