Calcium citrate is the calcium salt of citric acid. It is commonly used as a food additive (E333), usually as a preservative, but sometimes for flavor. In this sense, it is similar to sodium citrate. It is a dietary supplement that provides calcium, an essential mineral needed for many body functions. It’s commonly used to support bone health, help prevent osteoporosis, and support proper muscle and nerve function.
Calcium citrate is also found in some dietary calcium supplements (e.g. Citracal or Caltrate). Calcium makes up 24.1% of calcium citrate (anhydrous) and 21.1% of calcium citrate (tetrahydrate) by mass. The tetrahydrate occurs in nature as the mineral Earlandite.
Properties
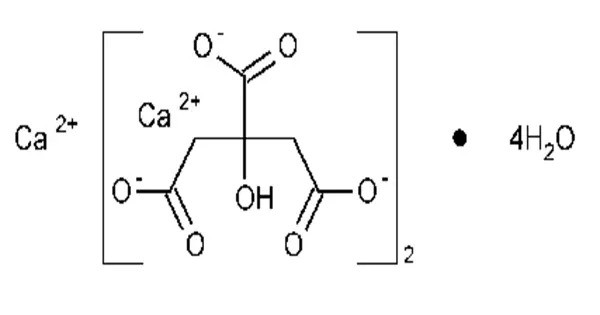
- Chemical formula: Ca3(C6H5O7)2
- Appearance: It typically appears as a white, odorless, crystalline powder.
- Solubility: It is more soluble in water than calcium carbonate, making it a preferred option in certain supplements because it is easier to absorb, especially for people with lower stomach acid.
- Molar Mass: The molar mass varies depending on the specific form, but it is typically around 498.2 g/mol for the tricalcium citrate form.
- pH: It is generally neutral to slightly basic when dissolved in water.
- Bioavailability: It has relatively high bioavailability compared to other calcium salts, meaning it is more easily absorbed by the body.
- Stability: It is stable under normal conditions and does not degrade easily.
- Molar mass: 498.4334 g/mol (anhydrous), 570.4945 g/mol (tetrahydrate)
- Odor: odourless
- Density: 1.63 g/cm3 (anhydrous), 2.00 g/cm3 (tetrahydrate)
- Melting point: Decomposes
- Boiling point: Decomposes
- Solubility in water: 0.85 g/L (18 °C), 0.95 g/L (25 °C)
- Solubility: insoluble in alcohol
Chemical properties
Calcium citrate is sparingly soluble in water. Needle-shaped crystals of tricalcium dicitrate tetrahydrate [Ca3(C6H5O7)2(H2O)2]·2H2O were obtained by hydrothermal synthesis. The crystal structure comprises a three-dimensional network in which eightfold coordinated Ca2+ cations are linked by citrate anions and hydrogen bonds between two non-coordinating crystal water molecules and two coordinating water molecules.
Production
Calcium citrate is an intermediate in the isolation of citric acid from the fungal fermentation process by which citric acid is produced industrially. The citric acid in the broth solution is neutralized by limewater, precipitating insoluble calcium citrate. This is then filtered off from the rest of the broth and washed to give clean calcium citrate.
3 Ca(OH)2(s) + 2 C6H8O7(l) → Ca3(C6H5O7)2(s) + 6 H2O(l)
The calcium citrate thus produced may be sold as-is, or it may be converted to citric acid using dilute sulfuric acid.
Natural Occurrence
Calcium citrate is naturally found in small amounts in various plants and foods, as citric acid (which combines with calcium to form calcium citrate) is common in many fruits, particularly citrus fruits like oranges, lemons, and grapefruits. However, calcium citrate itself is not found abundantly in nature as a pure compound.
Commercial Production
It is synthetically produced by reacting calcium carbonate (from limestone or other sources) with citric acid (often derived from citrus fruits). The process involves neutralizing citric acid with calcium hydroxide or calcium carbonate.
Uses
- Dietary Supplements: As mentioned, it’s most commonly used in calcium supplements, particularly because it can be taken with or without food and has less likelihood of causing gastrointestinal discomfort compared to other forms like calcium carbonate.
- Food Industry: Calcium citrate is used as a food additive, stabilizer, and preservative in some processed foods, especially beverages and dairy products.
- Medical Uses: In addition to calcium supplementation, it can be used as an alkalizing agent in certain medical treatments.