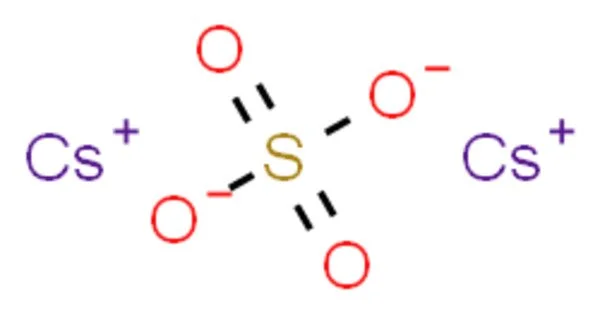
Caesium sulfate or cesium sulfate is the inorganic compound and salt with the formula Cs2SO4. It’s an inorganic salt that typically appears as a white or colorless crystalline solid. It is a white water-soluble solid that is used to prepare dense aqueous solutions for use in isopycnic (or “density-gradient”) centrifugation. It is isostructural with potassium salt. The compound is soluble in water, and it’s usually prepared by reacting caesium carbonate (Cs₂CO₃) or caesium hydroxide (CsOH) with sulfuric acid (H₂SO₄).
Caesium sulfate is notable for its use in various industrial applications, including in the production of caesium-based compounds, and in some specialized research fields like in the preparation of cesium salts used for atomic clocks, or in certain chemical syntheses.
Properties
It typically appears as a white, crystalline solid. It is highly soluble in water, forming a clear, colorless solution. It is relatively stable and does not undergo significant reactions under normal conditions, but it can react with strong acids to release sulfuric acid and caesium salts.
- Chemical formula: Cs2SO4
- Molar mass: 361.87 g/mol
- Density: 4.243 g/cm3, solid
- Melting point: 1,010 °C (1,850 °F; 1,280 K)
- Solubility in water: 167 g/100 ml (0 °C), 179 g/100 ml (20 °C)
- Solubility: insoluble in ethanol, acetone
Occurrences
- Natural Occurrence: Caesium sulfate is not found in large quantities in nature but can be obtained by reacting caesium compounds (such as caesium carbonate) with sulfuric acid.
- Production: It is often synthesized for use in laboratory and industrial applications, especially in the production of caesium salts and other caesium compounds.
Uses
- Production of caesium compounds: It serves as a precursor for other caesium-based chemicals.
- Vacuum tubes and photomultiplier tubes: Due to its high density and stability, caesium sulfate finds applications in some specialized industrial equipment.
- Isotope separation: Caesium sulfate has been researched for use in processes like isotopic separation, especially for caesium-137.